Proposal
(676) to South American Classification Committee
Split the
Sharp-beaked Ground-Finch (Geospiza
difficilis) and the Large Cactus-Finch (Geospiza
conirostris) into multiple species
Background
Although
work on speciation, hybridization, beak size evolution, and many other topics
have been studied in depth, and sometimes in novel ways in the Darwin’s
Finches, it is only recently that a modern phylogeny was produced for this
group of tanagers (Petren et al. 2005). It also has been clarified that the
Darwin’s Finches are part of the “dome nest clade” which includes Tiaris and various other largely
Caribbean taxa (Burns et al. 2002). However, even in recent molecular
phylogenies, populations from different islands were not sampled, leaving in
question whether morphologically unique populations were in fact also
genetically unique. Further, these molecular phylogenies have been based on
mtDNA and a few microsatellite loci.
New
Information
Lamichhaney
et al. (2015) have produced a new molecular phylogeny, including samples from
multiple islands for various species of Darwin’s Finches. This study is based
on a whole-genome re-sequencing of 120 individuals. They discovered evidence
for widespread historical gene flow between various populations of Darwin’s
Finches, and that genetic diversity is higher than would be expected in small
insular populations, likely through gene flow from other species. Their results
upheld the general phylogenies proposed recently; however, they differed in
finding that various populations of Geospiza
difficilis (Sharp-beaked Ground-Finch) and Geospiza conirostris (Large Cactus-Finch) are not sisters to each
other. They also discovered a locus associated with beak shape, and discussed
this in terms of ecology and evolution of members of this group. They concluded
that the group is approximately 1 million years old, and that certain branches,
such as the tree finches are rather recent (200,000 years).
Below
is the suggested phylogeny as well as a map of populations sampled.
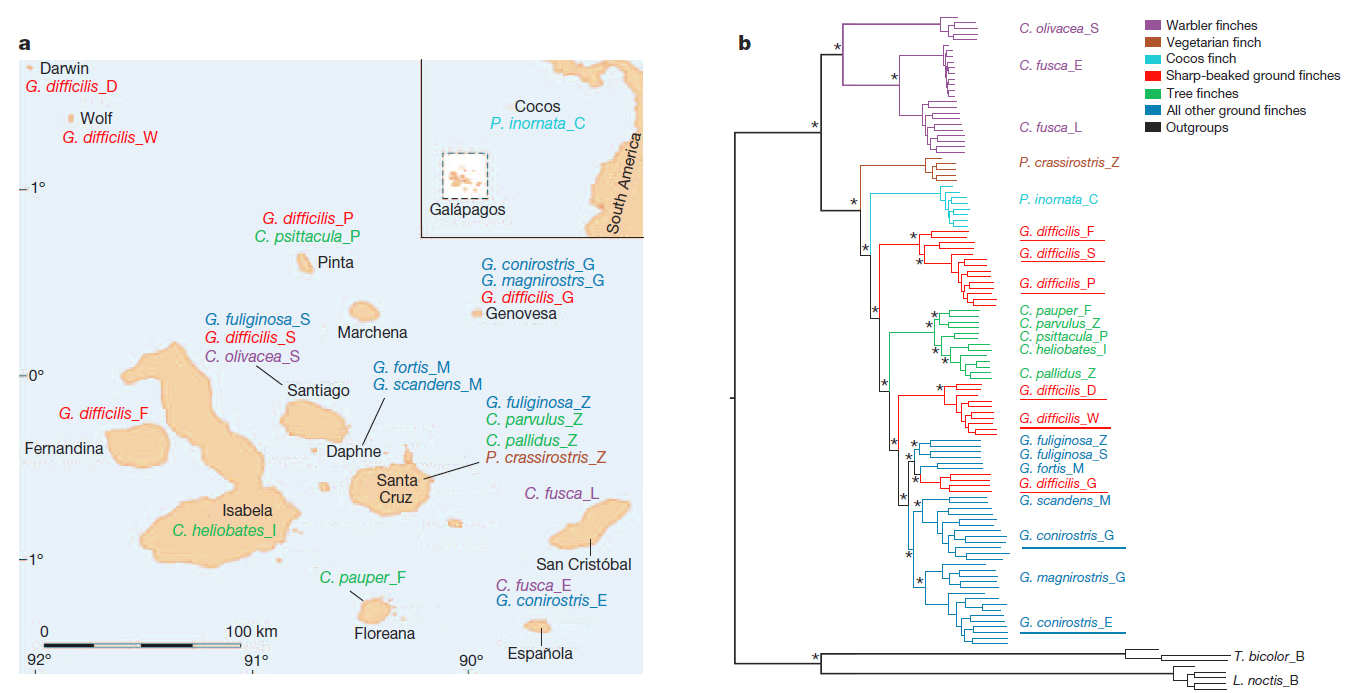
[Note
from Remsen: In the tree, difficilis
G[enovesa]= acutirostris;
difficilis F, S, P = nominate difficilis; difficilis D[arwin], W[olf] = septenttionalis; G.
conirostris G[enovesa] = propinqua; G. conirostris
E[spañola] = nominate conirostris.
Evidence for species splits
1) Geospiza difficilis,
Sharp-beaked Ground-Finch. Results confirm that there are three taxa widely
separated in the phylogeny. Three species level taxa are recommended to be
recognize:
G. acutirostris Ridgway; found on
Genovesa.
G. difficilis Sharpe; found on Pinta,
Fernandina, and Santiago.
G.
septentrionalis Rothschild and Hartert; found on Wolf and
Darwin.
These were formerly recognized as species,
based on differences in size and bill shape. The species difficilis has a straight culmen and is truly sharp beaked, whereas
septentrionalis has a curved culmen.
These populations also differ in song (Grant et al. 2000). The species acutirostris is very much smaller in
mass than the other two; in many ways it resembles a Small Ground-Finch (G. fuliginosa), and in fact it is
genetically much closer to fuliginosa
and fortis (Medium Ground-Finch) than
it is to true Sharp-beaked Ground-Finch. Curiously song is more similar to septentrionalis (Grant et al. 2000).
Lamichhaney et al. (20150 suggested that acutirostris
may be a species derived from mixed ancestry, i.e. of hybrid origin, but that
it is a distinct and separate entity (species).
2) Geospiza conirostris Large
Cactus-Finch. The two populations, one on Genovesa and one on Española, are
each genetically more similar to other species than they are to each other. The
Genovesa population (propinqua) is
sister to the Common Cactus-Finch (G.
scandens), whereas the nominate forma is sister to the Large Ground-Finch (G. magnirostris). This mirrors
morphology, both are big and large-billed, but propinqua has a long bill like a Cactus-Finch, whereas conirostris has a deep bill like a Large
Ground-Finch. The two forms of conirostris
differ in song, and song playbacks on Genovesa found only a weak attraction
effect when playing back songs from Española (Ratcliffe et al. 1985). Note that
there was absolutely no response from playback of Large Ground-Finch songs on conirostris. The playback results
between propinqua and Common
Cactus-Finch are more complex. The form propinqua
reacts strongly to one song type of Common Cactus-Finch from Daphne Major, but
Common Cactus-Finch does not react to songs of propinqua. Furthermore male Common Cactus-Finch discriminated
strongly against propinqua when
tested with a pair of museum specimen models in female plumage, one of propinqua and one of local scandens (Common Cactus): the reciprocal
experiment on Genovesa was not completed (Ratcliffe et al. 1983). Putting this
together, the evidence suggests that Common Cactus-Finch and propinqua, its sister species, would
rarely if ever interbreed if they came into contact. Note that there are old
records of Large Cactus-Finch from Darwin and Wolf islands. However, these are
based on only a few records and specimens. The current status of a population
on Darwin Island is unclear, and there are none currently on Wolf Island. The
subspecies darwini has been named,
although there is no evidence that there is a recent population from Darwin
Is., and in fact there has been confusion as it is thought that these may have
been stray Large Ground-Finches (Wiedenfeld 2006).
Recommendation
I
recommend a Yes vote to separate these species, raising the number of Darwin’s
Finch species from 15 to 18. Note that geographically the existence of a Large
Cactus-Finch on Darwin and Wolf islands is very unlikely, unless it also was a
separate and unique population. There is no recent evidence of a long-term
sustaining population on Wolf. It is unclear if the species is present and
common on Darwin Island, and indeed these may have been either stray Large
Ground-Finch, or perhaps a population of Large Cactus-Finch. Therefore, I think
it is best to delay any decision on what to do with darwini (if indeed it still exists), until genetic data from
specimens is studied. In the past darwini
has been lumped with propinqua.
English
Names
G.
acutirostris Ridgway;
found on Genovesa. – Restricted to Genovesa, why not Genovesa Ground-Finch? It is small and similar to Small
Ground-Finch, so a size or even a bill shape name does not jump out based on
its morphology.
G.
difficilis Sharpe;
found on Pinta, Fernandina and Santiago. – This is the most widespread, and
perhaps the archetype “Sharp-beaked Ground-Finch” so I would suggest letting it
retain the name Sharp-beaked
Ground-Finch.
G. septentrionalis Rothschild
and Hartert; found on Wolf and Darwin. Vampire
Ground-Finch, based on its well-known habit of feeding on booby blood. The
colloquial Vampire Finch has been in use for some time, but to be consistent I
think we would need to use Ground-Finch.
G.
conirostris; found on Española. This huge-billed bird is
most similar to the Large Ground-Finch, which it is sister to. I don’t know if
one can come up with a morphological based name, such as Thick-billed
Ground-Finch that mentions anything unique? Perhaps Española Ground-Finch would be the best name, because it is endemic
to that island.
G.
propinqua; found on Genovesa. This is sister to the Common
Cactus-Finch, so perhaps it should keep the name Large Cactus-Finch? Although this may be confusing as it is not any
more widespread or easily found than conirostris.
Genovesa Cactus-Finch would be
another possible name, noting that above we already have a Genovesa
Ground-Finch.
VOTING:
Subproposal
A – Accept separation of G. difficilis
into three species.
Subproposal
B – Accept separation of G. conirostris
into two species.
If
these above proposals pass, then we will consider proposals on English Names.
Literature
Cited
Burns, K. J., S.J.
Hackett and N. K. Klein. (2002). Phylogenetic relationships and morphological
diversity in Darwin’s Finches and their relatives. Evolution 56(6): 1240–1252
Grant, B. R., Grant, P.
R. & Petren, K. (2000). The allopatric phase of speciation: the sharp
beaked ground finch (Geospiza difficilis)
on the Galapagos islands. Biol. J. Linn. Soc. 69, 287–317.
Lamichhaney, S., J.
Berglund, M. Sällman Almén, M. Khurram, M. Grabherr, A. Martinez-Barrio, M.
Promerová, C.-J. Rubin, C. Wang, N. Zamani, B. R. Grant, P. R. Grant, M. T.
Webster and L. Andersson (2015). Evolution of Darwin’s finches and their beaks
revealed by genome sequencing. Nature 518: 371–375.
Petren, K., P.R. Grant,
B. R. Grant and L. F. Keller (2005). Comparative landscape genetics and the
adaptive radiation of Darwin’s finches: the role of peripheral isolation. Molecular
Ecology 14: 2943–2957
Ratcliffe, L. M. &
Grant, P. R. (1983). Species recognition in Darwin’s finches (Geospiza, Gould). II. Geographic
variation in mate preference. Anim. Behav. 31, 1154-1165.
Ratcliffe, L. M. &
Grant, P. R. (1985). Species recognition in Darwin’s finches (Geospiza, Gould). III. Male responses to
playback of different song types, dialects and heterospecific songs. Anim.
Behav. 31, 290-307.
Wiedenfeld, D. A.
(2006). Aves, The Galapagos Islands, Ecuador. Check List 2(2): 1-27.
Alvaro
Jaramillo, July 2015
=========================================================
Comments
from Zimmer: “YES to
Subproposal A and YES to Subproposal B, for reasons detailed by Alvaro in the
Proposal.”
Comments from Stiles:
“A)
YES. No problem here.
“B) YES, given
the more complete genetic sampling (all this makes me wonder about splitting up
the small, compact clade of Camarhynchus (?)
in green into all those species; presumably vocal evidence).”
Comments
from Pacheco: “YES to A
and YES to B. I am backing Alvaro’s two recommendations from molecular
phylogeny with samples geographically well distributed.”
Comments from Robbins: “NO. Having read the McKay and Zink (2015) paper
on Geospiza, in which they suggest
there is only a single species, one needs to pause and rethink things. So, we want to add yet more species to a
highly controversial Geospiza species
limits definition? For now, I vote no,
if for no other reason than to bring to the attention that the committee needs
to be aware of the latest perspective on this group.”
Additional comments from Robbins:
“With regard to the Geospiza proposal. The abstract of the Lamichhaney et al. (2015)
paper states the following:
‘Phylogenetic
analysis reveals important discrepancies with the phenotype-based taxonomy. We
find extensive evidence for interspecific gene flow throughout the radiation.
Hybridization has given rise to species of mixed ancestry.’
“Additionally, in the text on
the first page, ‘Extensive
sharing of genetic variation among populations was evident, particularly among
ground and tree finches, with almost no fixed differences between species in
each group (Extended Data Fig. 2).’ Finally, note that sampling is
certainly not extensive, so hybridization may well be underrepresented.
All of this should cause one to pause and think about the McKay and Zink
perspective. As we have appreciated throughout our careers, this a very
complex situation and it would be an understatement to say attempting to apply
traditional species limit views is difficult.
“I certainly would welcome
input from those who have a deep understanding of the genetics and analyses of
the Lamichhaney et al. (2015) results.”
Comments
from Remsen: “YES to A and B. Darwin’s finches continue to yield insights
into the complexity of the early stages of speciation. These populations have all diverged to the
point in terms of voice and behavior consistent with their treatment as BSC
species. In my view, that their mtDNA
has not yet sorted out reflects the lag time between these neutral loci versus
those under selection, or a degree of ongoing hybridization that does not
conflict with species rank (as in all hybridizing taxa that are also treated as
species under BSC).”
Comments
from Cadena: “YES.
The genomic data clearly point to problems with species delimitation, and
Alvaro has nicely summarized evidence showing the genomic data match well with
geography, morphology, vocalizations, and behavior. The paper mentioned by Mark
is indeed intriguing, but I did not find the analyses of morphological data in
that study entirely satisfying. In fact, with Felipe Zapata and Iván Jiménez,
we are working on re-analyses of morphological data using quantitative
approaches, and the results we get do not quite agree with the conclusions of
McKay and Zink based on their PCAs. We hope to write up a note describing this
soon.”