There are six color-based groups in the prasinus complex, within which some have
additional described subspecies. These major groups have been recognized
through much of the history of the taxon (Table 1) and were reaffirmed by the
analyses of del Hoyo and Collar (2014). The characters upon which they are
based are given in Winker (2016: table 2) and can be seen in the accompanying
Plate.
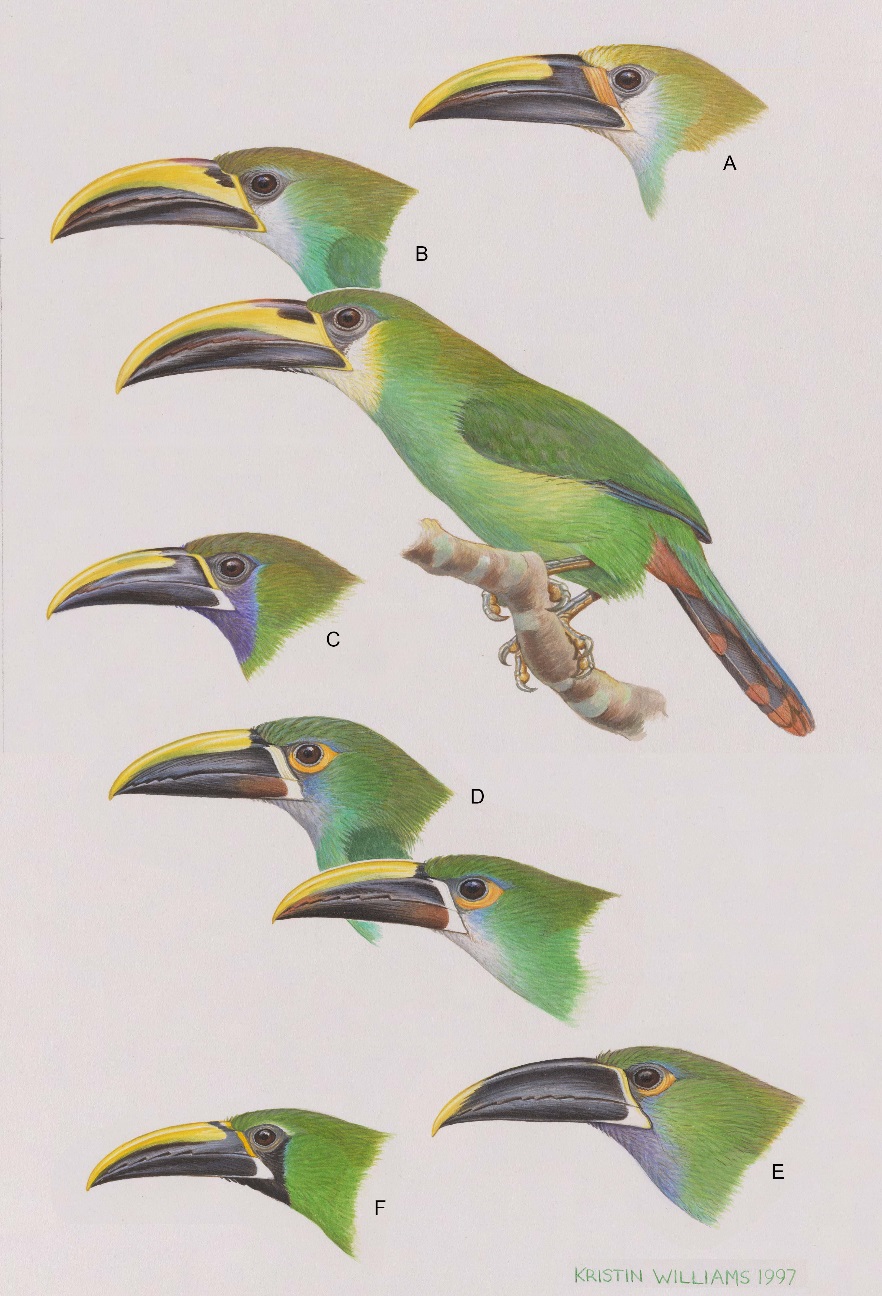 In Winker (2016) I tested the hypothesis that these are
“cookie-cutter” (i.e., morphologically nearly identical) toucanets differing
mostly in coloration. I also examined specimens carefully for phenotypic
evidence of hybridization.
In Winker (2016) I tested the hypothesis that these are
“cookie-cutter” (i.e., morphologically nearly identical) toucanets differing
mostly in coloration. I also examined specimens carefully for phenotypic
evidence of hybridization.
 A couple of key factors were central to my
treatment of the group. First, these birds move about considerably during the
nonbreeding season, providing hypothetical opportunities for gene flow across
zones of nearest approach. “For example, in south-central Mexico (Oaxaca), A. prasinus and A. wagleri breed within about 100 km of each other, a distance that
A. prasinus individuals appear to
move routinely away from their breeding areas, e.g., at the base of the Yucatan
Peninsula (e.g., Land, 1970; Jones, 2003), which does not seem unusual for an
arboreal frugivore (see also discussions in O’Neill & Gardner, 1974, and
Navarro et al., 2001).” (Winker 2016). The hitherto unrecognized (although
published by Puebla-Olivares et al. 2008) gene flow between albivitta and atrogularis in NE Ecuador indicates that this hypothesis has merit.
Second, I considered that the likelihood of successful gene flow/reticulation
between two lineages decreases with increased anagenesis or adaptive
divergence, arguing as follows (Winker 2016):
A couple of key factors were central to my
treatment of the group. First, these birds move about considerably during the
nonbreeding season, providing hypothetical opportunities for gene flow across
zones of nearest approach. “For example, in south-central Mexico (Oaxaca), A. prasinus and A. wagleri breed within about 100 km of each other, a distance that
A. prasinus individuals appear to
move routinely away from their breeding areas, e.g., at the base of the Yucatan
Peninsula (e.g., Land, 1970; Jones, 2003), which does not seem unusual for an
arboreal frugivore (see also discussions in O’Neill & Gardner, 1974, and
Navarro et al., 2001).” (Winker 2016). The hitherto unrecognized (although
published by Puebla-Olivares et al. 2008) gene flow between albivitta and atrogularis in NE Ecuador indicates that this hypothesis has merit.
Second, I considered that the likelihood of successful gene flow/reticulation
between two lineages decreases with increased anagenesis or adaptive
divergence, arguing as follows (Winker 2016):
“Effective lineage reticulation requires that
hybrid offspring have equal or greater fitness than offspring of pure parental
forms. Also, gene flow must occur frequently enough to overcome the
differentiating selective factors likely to be operating on largely allopatric
populations (and this relationship is nonlinear; see Winker, 2010 for
discussion). The more differences there are between populations in morphology,
the more differences there are likely to be in selective factors operating on
these populations and the more difficult effective gene flow is likely to be
between populations; at larger scales this results in the general correlation
between morphological difference and reproductive isolation (Mayr, 1963; Price,
2008).”
 Another important factor that I considered that did not
seem to have been adequately addressed before is that named subspecies in this
group do not represent equivalent levels of divergence. Historically, it seemed
that commonly observed intergradation between named forms within the major
color-based groups (among the more minor forms) led to observations that
hybridization was common, but this seemed to cloud a thorough understanding of
the full distribution of hybridization in the whole group, i.e., it is not just
where birds hybridize, but where they do not and what phenotypic
characteristics accompany these phenomena. I focused on the major groups and
made pairwise comparisons between them.
Another important factor that I considered that did not
seem to have been adequately addressed before is that named subspecies in this
group do not represent equivalent levels of divergence. Historically, it seemed
that commonly observed intergradation between named forms within the major
color-based groups (among the more minor forms) led to observations that
hybridization was common, but this seemed to cloud a thorough understanding of
the full distribution of hybridization in the whole group, i.e., it is not just
where birds hybridize, but where they do not and what phenotypic
characteristics accompany these phenomena. I focused on the major groups and
made pairwise comparisons between them.
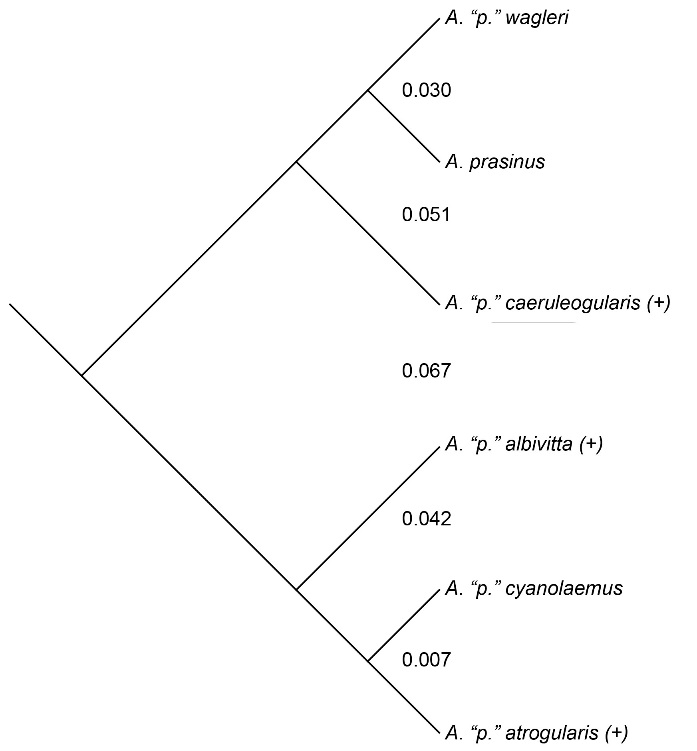 My results (from 578
specimens) showed multiple and complex morphometric relationships between
pairwise comparisons of neighboring forms. These differences were different
between the sexes and the differences were different between populations, and
only a small percentage of the variation observed could be explained by
geography (in females only, latitude and longitude explained < 6% of
variation). As it turned out, degrees of
morphometric differentiation were highly correlated with genetic distance (R2 = 0.67), as predicted by
the processes of anagenesis and speciation (Winker 2016: figure 5). Neither
geography nor phenotypic plasticity is likely to explain the degree of
differences found. “Concordant shifts in suites of mensural and other morphological
characters are precisely what we would predict to occur between individuals
representing genetically disjunct, locally adapted gene pools. Consequently,
this evidence suggests that this is what they are, and at these levels of
morphological differentiation (morphometrics, coloration, and pattern) we would
usually consider these groups to be full biological species.” But that conclusion
does not include consideration of hybridization.
My results (from 578
specimens) showed multiple and complex morphometric relationships between
pairwise comparisons of neighboring forms. These differences were different
between the sexes and the differences were different between populations, and
only a small percentage of the variation observed could be explained by
geography (in females only, latitude and longitude explained < 6% of
variation). As it turned out, degrees of
morphometric differentiation were highly correlated with genetic distance (R2 = 0.67), as predicted by
the processes of anagenesis and speciation (Winker 2016: figure 5). Neither
geography nor phenotypic plasticity is likely to explain the degree of
differences found. “Concordant shifts in suites of mensural and other morphological
characters are precisely what we would predict to occur between individuals
representing genetically disjunct, locally adapted gene pools. Consequently,
this evidence suggests that this is what they are, and at these levels of
morphological differentiation (morphometrics, coloration, and pattern) we would
usually consider these groups to be full biological species.” But that conclusion
does not include consideration of hybridization.
Evidence of hybridization between members of
the six color-based groups occurs phenotypically between cyanolaemus and atrogularis,
and (genetic evidence only) between atrogularis
and albivitta. The frequency of gene
flow was loosely inferred by using phenotypic evidence of hybridization as a
surrogate. Gene flow appears to be substantial between the two most closely
related taxa (0.7% divergence), cyanolaemus
and atrogularis, and rare (zero
phenotypic evidence) between albivitta
and atrogularis (4.2% divergence;
genetic data of Puebla-Olivares et al. 2008). There is no evidence for
Haldane’s rule occurring (genetic incompatibilities so extreme as to result in
higher levels of mortality in hybrids of the heterogametic sex—females in this
case). There was no evidence of hybridization among the North American forms
(3-5.1% divergence), nor between North and South American forms (6.7%
divergent).
“Hybridization per se is not sufficient evidence
for conspecificity, and in this group I find the lack of hybrids at most zones
of potential crossing of major subspecific groups to be more compelling in the
determination of species limits than its clear and seemingly routine presence
at one—particularly in light of the repeated evidence of varying suites of
morphological characters changing abruptly across these zones. However, I do
consider that the apparent frequency of hybridization between A. atrogularis cyanolaemus and A. a. atrogularis warrants a
conservative approach to their separation at the species level, and thus I do
not recommend doing so without more evidence. In short, morphologically there
is no evidence for hybridization between five of the major subspecific groups,
despite likely opportunity, especially in northern Middle America. This is
coupled with pronounced morphometric differences between these groups,
suggesting group-specific ecological adaptation in addition to whatever social
selection factors have likely caused the rather dramatic head and bill color
differences.”
(Winker 2016). In other words, I doubt these taxa exist in total allopatry, and
the genetic evidence between albivitta
and atrogularis would seem to support
this supposition, yet intergroup hybrids seem to be rare except between the two
most closely related forms, cyanolaemus
and atrogularis.
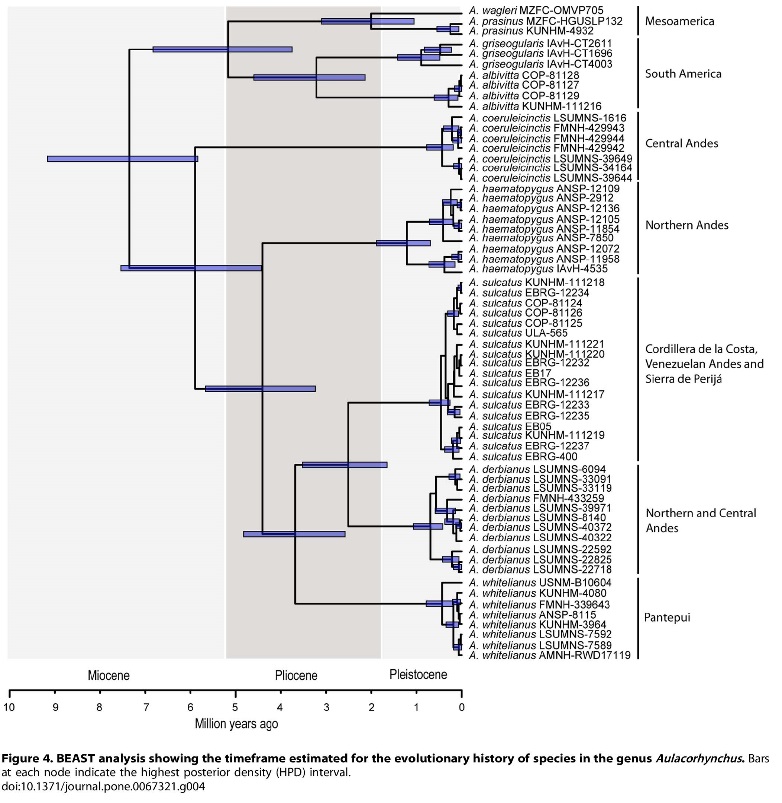 Voice is an important reproductive isolating mechanism
(RIM) in at least some Aulacorhynchus,
(Schwartz 1972, Haffer 1974). However, I think it would be a mistake to
consider it the only or even the most important one, despite its utility in
some cases. In Winker (2016) I did not discuss RIMs, but the treatment relied
more on the likelihood of postzygotic RIMs (increasing evidence of
morphological divergence making successful hybrids and reticulation less
likely) than on prezygotic ones (of which voice could be an important one).
From a subjective view, vocal divergence does not seem to be evolving as
quickly in the prasinus complex as it
has among other Aulacorhynchus
species in South America. The South American radiation of the species haematopygus, whitelianus, derbianus,
and sulcatus likely began after that
of the prasinus clade (~4.5 Mya vs. ~5.2
Mya; Bonaccorso et al. 2013, figure inserted here). But (subjectively) in the
former group vocal divergence has been more rapid (Schwartz 1972).
Voice is an important reproductive isolating mechanism
(RIM) in at least some Aulacorhynchus,
(Schwartz 1972, Haffer 1974). However, I think it would be a mistake to
consider it the only or even the most important one, despite its utility in
some cases. In Winker (2016) I did not discuss RIMs, but the treatment relied
more on the likelihood of postzygotic RIMs (increasing evidence of
morphological divergence making successful hybrids and reticulation less
likely) than on prezygotic ones (of which voice could be an important one).
From a subjective view, vocal divergence does not seem to be evolving as
quickly in the prasinus complex as it
has among other Aulacorhynchus
species in South America. The South American radiation of the species haematopygus, whitelianus, derbianus,
and sulcatus likely began after that
of the prasinus clade (~4.5 Mya vs. ~5.2
Mya; Bonaccorso et al. 2013, figure inserted here). But (subjectively) in the
former group vocal divergence has been more rapid (Schwartz 1972).
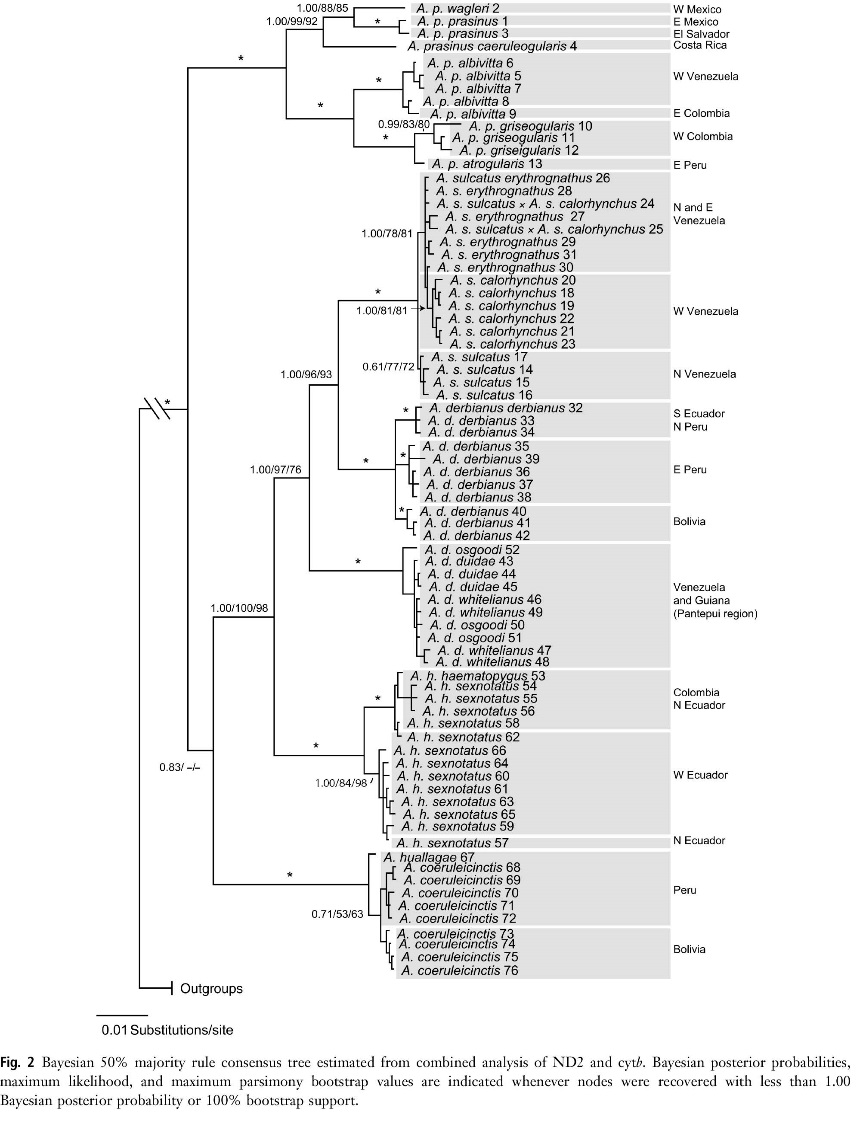 Donegan et al. (2015)
relied exclusively on voice in maintaining all prasinus taxa as one species, mostly reiterating prior work (though
providing more sonograms) of Haffer (1974) and Short and Horne (2001), which
downplayed phenotypic differences (not adequately explored, in my view) and
relied rather heavily on voice. Inadequate attention has been paid to the fact
that the vocally similar taxa hybridizing to a degree to be considered
conspecific (e.g., cyanolaemus-atrogularis
and sulcatus-calorhynchus; Schwartz
1972) are among the most closely related in the genus (Puebla-Olivares 2008,
Bonaccorso et al. 2011: fig. 2, inserted at right). And, again, there are
additional quite striking morphological characters changing besides bill and
throat colors. In addition to the mensural characteristics found in Winker
(2016), there are characters like eye-skin color changes and the basal upper
mandible encrustations in adult wagleri
that increase the likelihood of other RIMs being present in the absence of
vocal differences. So, despite vocal similarities among prasinus taxa, I consider the steadily increasing morphological
differences with increasing genetic distance (Winker 2016: fig. 5) and the
absence of phenotypic evidence of hybridization across most zones of closest
approach to warrant species-level splits.
Donegan et al. (2015)
relied exclusively on voice in maintaining all prasinus taxa as one species, mostly reiterating prior work (though
providing more sonograms) of Haffer (1974) and Short and Horne (2001), which
downplayed phenotypic differences (not adequately explored, in my view) and
relied rather heavily on voice. Inadequate attention has been paid to the fact
that the vocally similar taxa hybridizing to a degree to be considered
conspecific (e.g., cyanolaemus-atrogularis
and sulcatus-calorhynchus; Schwartz
1972) are among the most closely related in the genus (Puebla-Olivares 2008,
Bonaccorso et al. 2011: fig. 2, inserted at right). And, again, there are
additional quite striking morphological characters changing besides bill and
throat colors. In addition to the mensural characteristics found in Winker
(2016), there are characters like eye-skin color changes and the basal upper
mandible encrustations in adult wagleri
that increase the likelihood of other RIMs being present in the absence of
vocal differences. So, despite vocal similarities among prasinus taxa, I consider the steadily increasing morphological
differences with increasing genetic distance (Winker 2016: fig. 5) and the
absence of phenotypic evidence of hybridization across most zones of closest
approach to warrant species-level splits.
More work is needed in this group. Voice, for
example, although notably similar throughout the prasinus complex’s range (Haffer 1974, Donegan et al. 2015), does
show some likely pace differences between wagleri
and prasinus (Winker 2016). Also,
given the current evidence it seems likely that population genetic studies will
show low rates of historic gene flow across more of the zones of closest
contact.
“Using the
biological species concept, I suggest that consideration of all of the
available evidence indicates that we should recognize five species in the A. “prasinus”
complex (A. wagleri, prasinus,
caeruleogularis, albivitta, and atrogularis),
each with any associated named subspecies (Appendix).”
South American forms, where all of the
hybridization thus far recognized (between the major color-based groups) occurs,
remain the least certain, and future work may change the perceptions outlined
here.
Recommendation: Unsurprisingly, I
recommend voting Yes on all of A-G below (A, B, E, F, and G for NACC, and C, D,
E, F, and G for SACC).
For now, I will include in the proposal an up
or down vote on the English names given in the Appendix of Winker (2016).
Should either of those two votes fail while the split votes pass (NACC or
SACC), we will need to further address those issues.
NACC:
A) A yes vote would recognize all three major
Middle American forms (prasinus, wagleri, and caeruleogularis) as full biological species. [DID NOT PASS]
B) Should the vote on A pass, we need to adopt
English names for these taxa. A yes vote here would accept the English names
for these taxa proposed in Winker (2016), i.e., Northern Emerald Toucanet (A. prasinus), Wagler’s
Toucanet (A. wagleri), and
Blue-throated Toucanet (A.
caeruleogularis). The only change from historic usage is in adding
“Northern” to the first. Different historic treatments are given below in Table
2. [Northern Emerald-Toucanet was name adopted for the
Middle American species]
(More NACC below...)
SACC:
C) A yes vote would recognize two South
American forms (albivitta and atrogularis) as full biological species.
D) Should the vote on C pass, we need to adopt
English names for these taxa. A yes vote here would accept the English names
for these taxa proposed by Winker (2016), i.e., Southern Emerald Toucanet (A. albivitta) and Black-throated Toucanet
(A. atrogularis). The first gets
around throat-color problems both within the group and with the fact that the
white color of the nominate form’s throat matches that of prasinus sensu stricto. The second, however, does not, in that the
subspecies cyanolaemus has a blue
throat. Different historic treatments are given below in Table 2.
NACC and
SACC:
E) Should the “A” and/or “C” votes above fail
and we do not agree to recognize three and/or two species in each clade,
respectively, it occurs to me that we should at least split the group into the
two major clades, prasinus (North
America) and albivitta (South
America). Their nearest-approach neighbors in Panama and Colombia are
phenotypically and genetically the most divergent, and they’ve been apart for a
long time: an estimated ~1.7 Mya (using the 2% rule on the mtDNA data of
Puebla-Olivares et al. 2008) or ~5.2 Mya from Bonaccorso et al. (2013). For a
visual, see C and D in the accompanying Plate (Fig. 1 in the proposal) and the
specimen photograph inserted below (Fig. 2). A yes vote here would, if the A
and/or C votes above fail, recognize just two species in the prasinus complex, whose English names
might be...
F) Northern Emerald-Toucanet
(A. prasinus) and Southern Emerald-Toucanet (A. albivitta).
A yes vote here would accept these names should we only agree to split the
complex into two species.
G) Finally, I propose that we adopt the
sequence of taxa given in Winker (2016: appendix, copied below), which follows
both geography (N-S) and taxonomy and can be taxonomically adjusted to
accommodate the votes above.
Table 2. English names for prasinus taxa.
|
Cory
1919 |
|||
|
|
(names
all subspp.) |
HBW
2014 |
Winker
2016 |
|
A.
prasinus |
Emerald,
Southern Emerald |
Emerald
Toucanet |
Northern
Emerald Toucanet |
|
A.
wagleri |
Wagler's Toucanet |
Wagler's Toucanet |
Wagler's Toucanet |
|
A.
caeruleogularis |
Blue-throated,
Goldman's Bl-thr. |
Blue-throated |
Blue-throated
Toucanet |
|
A.
albivitta |
White-throated,
Grayish-blue-throated, Plumbeous-throated |
Grayish-throated |
Southern
Emerald Toucanet |
|
A.
griseigularis |
|||
|
A.
lautus |
Santa
Marta Toucanet |
(subsp.
of albivitta) |
|
|
A.
cyanolaemus |
Gray-throated
Toucanet |
Black-billed |
(subsp.
of atrogularis) |
|
A.
dimidiatus |
Ridgway's
Toucanet |
(subsp.
of atrogularis) |
|
|
A.
atrogularis |
Black-throated |
Black-throated |
Black-throated
Toucanet |

Figure 2 (only in proposal). Typical males of caeruleogularis (LSU 104668) and A. albivitta lautus (LSU 90407), the
most proximal North and South American forms.
Appendix (from
Winker 2016)
Suggested taxonomy.—Because I have
examined all of the described taxa in the complex, this revision includes
subspecies (although quantitative analyses were not undertaken below the level
of the six major groups). Given below are species, subspecies, authors of
original descriptions, type localities, and notes pertaining to each species.
Distribution is not included, because I did not examine all existing specimens
and can add little of substance to distributions set forth by the authors cited
herein. The species sequence given follows the relationships in the mtDNA tree
of Puebla-Olivares et al. (2008) but with the two major clades flipped to
better accommodate the group’s geographic distribution (as I have also done in
Fig. 4).
Genus
Aulacorhynchus
(green toucanets), subgenus Ramphoxanthus
Aulacorhynchus wagleri (Sturm in Gould 1841:pl. 16 (heft 2, pl. 6)). Wagler’s Toucanet. no type loc. [= Guerrero and Oaxaca,
Mexico].
Aulacorhynchus prasinus (Gould 1833). Northern
Emerald Toucanet.
A. p.
prasinus (Gould 1833). Mexico [= Valle Real, Oaxaca].
A. p.
warneri Winker (2000). Volcán San Martín,
Sierra de Los Tuxtlas, Veracruz, Mexico.
A. p.
virescens Ridgway (1912:88). Chasniguas,
Honduras.
A. p.
volcanius Dickey and van Rossem (1930:53).
Volcán de San Miguel, Dept. San Miguel, El Salvador.
Notes: A. p. stenorhabdus (Dickey and van Rossem
1930:52) and A. p. chiapensis
(Brodkorb 1940) are considered synonyms of A.
p. virescens; variation among them appears to be clinal (see also Monroe
1968). Wetmore (1941, notes in USNM) considered chiapensis as “doubtfully separable,” but recognized stenorhabdus. See
notes under A. albivitta regarding
the English common name.
Aulacorhynchus caeruleogularis (Gould 1854:45).
Blue-throated Toucanet.
A. c.
caeruleogularis
(Gould 1854:45). Veragua [, Panama] [= Boquete,
Chiriquí; Wetmore 1968:508].
A. c.
cognatus
(Nelson 1912:4). Mount Pirri (at 5,000 feet altitude)
head of Rio Limon, eastern Panama.
Notes: A. c. maxillaris
(Griscom 1924:2) is considered a synonym of A.
c. caeruleogularis (cf. Wetmore 1968:509). See Wetmore (1968) for citation
of the name caeruleogularis appearing
first in the Zoologist in 1853; no description appears there, however, the
reference being a report of what occurred at two meetings in February 1853
(“D.W.M.” 1853). Olson (1997) provided more notes on these occurrences in
relation to Gould.
Aulacorhynchus albivitta (Boissonneau 1840:70).
Southern Emerald Toucanet.
A. a.
lautus
(Bangs 1898:173). San Miguel [Sierra Nevada de Santa Marta], Colombia.
A. a.
griseigularis
Chapman (1915:639). Santa Elena (alt. 9000 ft.), Cen. Andes, Antioquia, Col.
A. a.
phaeolaemus
Gould (1874:184). Concordia, in Columbia [sic], and Merida, in Venezuela [=
Concordia, Antioquia, western Colombia; Hellmayr 1911:1213].
A. a.
albivitta
(Boissonneau 1840:70). Santa-Fé de Bogota [,
Colombia].
Notes: Chapman (1917) inexplicably
omitted the occurrence of the species (endemic subsp. lautus) in the Santa Marta region. More detailed study is needed to
resolve problems in the status, relationship, distributions, and nomenclature
of phaeolaemus and griseigularis (see Chapman 1917, Haffer
1974). The English name for this species given by Cory (1919:377),
White-throated Toucanet, is only appropriate for the subspecies albivitta, and thus is more appropriate
at the species level for A. prasinus
(sensu stricto, though not used
there). The other subspecies of albivitta
are all grayish or grayish-blue on the throat. Del Hoyo and Collar (2014)
suggested Grayish-throated, but this overlooks both white-throated birds and
those with blue in the throats. Accordingly, I have suggested more fitting
English names for this species and A.
prasinus.
Aulacorhynchus atrogularis (Sturm in Gould 1841:heft 2, pl.2 & text).
Black-throated Toucanet.
A. a.
cyanolaemus
(Gould 1866:24). Loxa [=Loja] in Ecuador.
A. a.
atrogularis
(Sturm in Gould 1841:heft 2, pl.2
& text). Andes of Peru [=Chunchamayo, central
Peru; Cory 1919:380).
A. a.
dimidiatus
(Ridgway 1886:93). No loc.; suggested by O'Neill and Gardner (1974:703) to be
along the eastern foothills of the Andes of central southern Peru.
Note: Recognition of A. a. dimidiatus follows O'Neill and
Gardner (1974). A. a. cyanolaemus is
blue-throated (Fig. 1).
Literature Cited
American
Ornithologists’ Union (AOU). 1983. Check-list
of North American birds (6th ed). Lawrence, Kansas: American Ornithologists’
Union.
American
Ornithologists’ Union (AOU). 1998. Check-list
of North American birds (7th ed). Washington, D. C.: American
Ornithologists’ Union.
Avise J, Wollenberg K. 1997. Phylogenetics and the origin of
species. Proceedings of the National
Academy of Sciences USA 94:7748-7755.
Bangs, O.
1898. On some birds from the Sierra Nevada de Santa Marta, Colombia. Proceedings of the Biological Society of
Washington 12:171-182.
Boissonneau
M. 1840. Oiseaux nouveaux de Santa-Fé de Bogota. Revue Zoologique
1840:66-71.
Bonaccorso
E, Guayasamin JM, Peterson AT, Navarro-Sigüenza AG.
2011. Molecular phylogeny and systematics of Neotropical toucanets in the genus
Aulacorhynchus. Zoologica Scripta 40:336-349.
Brabourne
L, Chubb C. 1912. The birds of South America.
Vol. I. London: Taylor and Francis.
Brodkorb P.
1940. New birds from southern Mexico. Auk
57:542-549.
Carriker MA
Jr. 1933. Descriptions of new birds from Peru, with notes on other little-known
species. Proceedings of the Academy of
Natural Sciences of Philadelphia 85:1-38.
Chapman FM.
1915. Diagnoses of apparently new Colombian birds. IV. Bulletin of the American Museum of Natural History 34:635-662.
Chapman FM.
1917. The distribution of bird-life in Colombia: A contribution to a biological
survey of South America. Bulletin of the
American Museum of Natural History 36:1-729.
Cheviron
ZA, Brumfield RT. 2009. Migration-selection balance and local adaptation of
mitochondrial haplotypes in rufous-collared sparrows (Zonotrichia capensis) along an elevational gradient. Evolution 63: 1593-1605.
Cory CB.
1919. Catalogue of birds of the Americas. Part II, No. 2. Field Museum of Natural History Zoological Series 13:317-607.
Degnan JH,
Rosenberg NA. 2006. Discordance of species trees with their most likely gene
trees. PLoS Genetics 2:e68.
del Hoyo J,
Collar NJ. 2014. HBW and BirdLife
International Illustrated Checklist of the Birds of the World, Volume 1,
Non-passerines. Barcelona: Lynx Edicions.
"D. W.
M." 1853. Proceedings of the Zoological Society [being a report of two
meetings in February of this year]. Zoologist
1853:3860-3861.
Dickey DR,
van Rossem AJ. 1930. Geographic variation in Aulacorhynchus prasinus (Gould). Ibis 1930:48-55.
Dickinson
EC, and Remsen JV Jr. (Eds.) 2013. The
Howard and Moore Complete Checklist of the Birds of the World, 4th
ed., Volume 1 Non-Passerines. Eastbourne, U. K.: Aves Press.
Donegan T,
Quevedo A, Verhelst JC, Cortés-Herrera O, Ellery T, Salaman P. 2015. Revision
of the status of bird species occurring or reported in Colombia 2015, with
discussion of BirdLife International’s new taxonomy. Conservación Colombiana
23:3-48.
Dolman G,
Joseph L. 2015. Evolutionary history of birds across southern Australia:
structure, history and taxonomic implications of mitochondrial DNA diversity in
an ecologically diverse suite of species. Emu
115:35-48.
Funk DJ,
Omland K. 2003. Species-level paraphyly and polyphyly: Frequency, causes, and
consequences, with insights from animal mitochondrial DNA. Annual Review of Ecology Evolution and Systematics 34: 397-423.
Galtier N, Nabholz B, Glemin S, Hurst GDD. 2009. Mitochondrial DNA as a marker of
molecular diversity: a reappraisal. Molecular
Ecology 18: 4541-4550.
Gould J.
1833. A Monograph of the Ramphastidae, or
Family of Toucans (part 1). London: The author.
Gould J.
1841-47. Monographie der Ramphastiden.
Nuremburg.
Gould J.
1854. Description of a new species of Aulacorhamphus. Proceedings
of the Zoological Society of London 1853:45.
Gould J.
1866. Description of a new species of toucan belonging to the genus Aulacoramphus. Proceedings of the Zoological Society of London
1866:24.
Gould J.
1874. On three new species of toucans pertaining to the genus Aulacorhamphus. Annals & Magazine of Natural History (4)
14:183-4.
Griscom L.
1924. Descriptions of new birds from Panama and Costa Rica. American Museum Novitates 141:1-12.
Haffer J.
1974. Avian speciation in tropical South America. Publications of the Nuttall Ornithological Club 14:1-390.
Hellmayr
CE. 1911. A contribution to the ornithology of western Colombia. Proceedings of the Zoological Society of
London 1911:1084-1213.
Hilty SL,
Brown WL. 1986. A Guide to the Birds of
Colombia. Princeton University Press, Princeton, New Jersey.
Irwin D J.
2002. Phylogeographic breaks without geographic barriers to gene flow. Evolution 56: 2383-2394.
Jones HL.
2003. Birds of Belize. Austin:
University of Texas Press.
Land H.
1970. Birds of Guatemala. Wynnewood,
Pennsylvania: Livingston Publishing Company.
Mayr E.
1963. Animal Species and Evolution.
Cambridge, Massachusetts: Belknap Press.
Morales HE,
Pavlova A, Joseph L, Sunnucks P. 2015. Positive and purifying selection in
mitochondrial genomes of a bird with mitonuclear discordance. Molecular Ecology 24:2820-2837.
Navarro S
AG, Peterson AT, López-Medrano E, Benítez-Díaz H. 2001. Species limits in
Mesoamerican Aulacorhynchus
toucanets. Wilson Bulletin
113:363-372.
Nelson EW.
1912. Descriptions of new genera, species and subspecies of birds from Panama,
Colombia and Ecuador. Smithsonian
Miscellaneous Collections 60:1-26.
Olson SL.
1997. [Review of] John Gould the Bird Man.
Auk 114:540-541.
O’Neill JP,
Gardner AL. 1974. Rediscovery of Aulacorhynchus
dimidiatus (Ridgway). Auk
91:700-704.
Pavlova A,
Amos JN, Joseph L, Loynes K, Austin J, Keogh JS,
Stone GN, Nicholls JA, Sunnucks P. 2013. Perched at the mito-nuclear
crossroads: divergent mitochondrial lineages correlate with environment in the
face of ongoing nuclear gene flow in an Australian bird. Evolution 67:3412-3428.
Peters JL.
1948. Check-list of birds of the world,
Vol. VI. Cambridge, Massachusetts: Harvard University Press.
Peters J L,
Winker K, Millam KC, Lavretsky P, Kulikova
I, Wilson RE, Zhuravlev YN, McCracken KG. 2014.
Mito-nuclear discord in six congeneric lineages of Holarctic ducks (genus Anas).
Molecular Ecology 23:2961-2974.
Price, T.
2008. Speciation in Birds. Greenwood
Village, Colorado: Roberts and Company.
Puebla-Olivares
F, Bonaccorso E, Espinosa de los Monteros A, Omland KE, Llorente-Bosquets
JE, Peterson AT, Navarro-Sigüenza AG. 2008. Speciation in the emerald toucanet
(Aulacorhynchus prasinus) complex. Auk 125:39-50.
Remsen JV
Jr, Areta JI, Cadena CD, Jaramillo A, Nores M, Pacheco JF, Pérez-Emán J,
Robbins MB, Stiles FG, Stotz DF, Zimmer KJ. Version 14 April 2016. A
classification of the bird species of South America. American Ornithologists’
Union. www.museum.lsu.edu/~Remsen/SACCBaseline.html
Ribeiro AM,
Lloyd P, Bowie RCK. 2011. A tight balance between natural selection and gene
flow in a southern African arid-zone endemic bird. Evolution 65:3499-3514.
Ridgway R.
1886. Descriptions of some new species of birds supposed to be from the
interior of Venezuela. Proceedings of the
U. S. National Museum 9:92-94.
Ridgway R.
1912. Descriptions of some new species and subspecies of birds from tropical
America. Proceedings of the Biological
Society of Washington 25:87-92.
Salvin O,
Godman FD. 1896. Biologia Centrali-Americana, Aves. Vol. II. London: Taylor and
Francis.
Sclater PL.
1891. Family Rhamphastidae, pp. 122-160 in Catalogue of the Birds in the British
Museum, Volume XIX. London: British Museum (Natural History).
Short LL.,
Horne JFM. 2001. Toucans, barbets, and
honeyguides. Oxford: Oxford University Press.
Sibley CG,
Monroe BL Jr. 1990. Distribution and
taxonomy of birds of the world. New Haven, Connecticut: Yale University
Press.
Toews DP,
Brelsford A. 2012. The biogeography of mitochondrial and nuclear discordance in
animals. Molecular Ecology 21:3907-3930.
Wetmore A.
1968. The birds of the Republic of
Panama. Part 2. Columbidae (pigeons) to Picidae (woodpeckers). Washington,
D. C.: Smithsonian Institution Press.
Winker K.
2000. A new subspecies of toucanet (Aulacorhynchus
prasinus) from Veracruz, Mexico. Ornitología
Neotropical 11:253-257.
Winker K. 2016. An examination of
species limits in the Aulacorhynchus “prasinus” toucanet complex (Aves:
Ramphastidae). PeerJ 4: e2381 https://doi.org/10.7717/peerj.2381.
Winker K.
2010. Subspecies represent geographically partitioned variation, a goldmine of
evolutionary biology, and a challenge for conservation. Ornithological Monographs 67:6-23.
Kevin Winker, February
2018
Remsen: Synopsis for
SACC voting:
A. Split Emerald
Toucanet into two species, i.e. Aulacorhynchus
prasinus (Middle America) Aulacorhynchus
albivitta (South America). (This was
adopted by NACC.)
B. Adopt English names Northern
Emerald-Toucanet and Southern Emerald-Toucanet for the two species above. (A NO vote indicates you have a better
alternative or that you voted NO on C.)
C. Split South American
populations into two species, i.e. Aulacorhynchus
albivitta (Colombia and Venezuela) and A.
atrogularis (Ecuador to Bolivia).
D. Adopt English names Southern
Emerald-Toucanet and Black-throated Emerald-Toucanet for the two species
above. Note that the group name
Emerald-Toucanet was not used for atrogularis
in the original proposal, but this would be required under our
conventions. (A NO vote indicates you
have a better alternative or that you voted NO on C.)
__________________________________________________________
Comments from Stiles:
“A. YES - splitting the complex into two species, prasinus and albivitta, seems the best description as the evidence now stands.
“B. YES, these English names go well with this split.”
“C. NO - this split is not justified due to the close genetic
relationship and apparently frequent hybridization between these taxa.
Comments
from Robbins:
“A.
YES, for elevating albivitta to
species level.
“C.
NO, until more data become available.”
Comments from Pacheco:
“A) YES.
“C) YES. I think the data presented by Winker allow the adoption of this split.”
Comments from Remsen: “NO on all. Here are my comments from the NACC proposal,
which I stand behind:
Remsen: NO.
This is a tough one, I’ve gone back and forth, and I really appreciate
Kevin’s effort and analysis on this proposal.
Although some of the rationale presented is reasonably convincing
concerning the accumulated phenotypic differences among these taxa as likely
being RIMs, I can’t get past the following points:
1. In the one “test case” in
which we have two parapatric taxa, cyanolaemus
and atrogularis hybridize freely ….
or do they? As Kevin noted,
hybridization per se among BSC species is acceptable, but whether this is the
case with these two is either not known or not made specific in the
proposal. Lacking time and energy to
investigate this myself thoroughly, I am going to assume that the signal from
the contact zone is that there are no pure parentals, i.e. nonassortative
mating (because Kevin considered them conspecific). If that is the case, then empirically we
conclude that strong differences in color of the bill, throat, and facial skin
are not RIMs in the “test case”. That’s
a lot of “ifs” but that’s all I have to go on.
By perilous extrapolation to the allotaxa, none of those phenotypic
differences can be used as reasons for recognition of taxa that differ in all
three of those characters, much less just one or two. I emphasize that that this string of
conditional statements rests on what is likely an imperfect knowledge of the
contact zone and thus easily reversed with more data.
2. Outside the prasinus group but within Aulacorhynchus, the other test case of
parapatric taxa is calorhynchus vs. sulcatus, which was studied by Schwartz (1972).
They differ dramatically in bill color but are similar in throat and
face color, and there are no vocal differences.
The contact zone sends a clear signal: nonassortative mating (nothing
but intermediate birds). Therefore, by
extrapolation, major differences in bill color among allotaxa are not
sufficient evidence for species rank (with all the obvious caveats concerning
such extrapolations … but it’s all we have to go on.). (By the way, HBW treats these two as separate
species despite free interbreeding, in part because in their scoring scheme,
hybridization counts as a “+1” point towards considering the two as separate
species – go figure …. ).
3. Outside Aulacorhynchus, in Pteroglossus and Ramphastos,
Haffer has shown that major differences in plumage and bill color do not serve
as RIMs. Although none of the contact
zones has been sampled as thoroughly as we would like, the signal sent from the
contact zones is existence of hybrid swarms and lack of RIMs. Therefore, in Ramphastidae as a whole,
divergence in plumage and bill color does not insure that these populations are
evolving independently. In contrast,
Haffer showed that sympatric species from different lineages within Ramphastos are more similar to each
other in plumage and bill color than they are to their own closest relatives,
i.e. plumage and bill color make no difference in their treatment as separate
species. However, as Haffer also showed,
vocal differences were clear predictors of genetic isolation.
In summary, although I
appreciate Kevin’s point that voice should not be regarded a priori as the only character that indicates species status, in
this particular group, the limited empirical data indicate that vocal
differences predict absence of free gene flow, whereas coloration patterns are
irrelevant. So, if our species concept
focuses on free gene flow, or lack of it, then vocal differences or lack of
them should indeed be the criterion by which we assign rank to allopatric taxa. Recognizing that we all know the dangers of
such extrapolations due to the serendipitous nature of speciation, I
nonetheless see no alternatives other than whimsy. As noted by Donegan et al. (2015), all
members of the prasinus group are, as
far as is known, vocally extremely similar if not indistinguishable, and
therefore, within the comparative framework of what we know about gene flow
between toucan populations, this lack of difference indicates lack of
divergence to the level associated with known cases of assortatively mating
toucans, i.e., we should treat all taxa as subspecies pending further data.
The only nit-picky problem I find with
Kevin’s rationale is use of nonbreeding dispersal anecdotes to predict
opportunities for gene flow. Similar
rationale was woven into the recent Willet proposal, in which one taxon’s
nonbreeding range overlaps with the breeding range of the other. I did not look up the particulars, but the
significance of a 100 km dispersal event depends on context. i.e. whether it is
within the habitat and range of the species versus whether it crosses a true
barrier to dispersal. Of course greater
vagility indicates greater long-term prospects for gene flow, which is probably
all Kevin was implying, but I do not think it should count in any taxonomic
interpretation. Also, genetic evidence
of hybridization could reflect past conditions in which the two taxa were
closer (versus contemporary dispersal).
Incidentally, “Northern Emerald
Toucanet” and “Southern Emerald Toucanet” are unacceptable in my opinion
because, even without hyphens, these names imply that they are sister taxa,
which is clearly incorrect. HBW avoided
this by leaving prasinus as Emerald
Toucanet and using “Greyish-throated Toucanet” for albivitta, which as Kevin points out is not really appropriate either. If the proposal passes, I strongly recommend
pulling out the English name sections as a separate proposal and investigating
alternatives.
Comments from Claramunt:
“A. Yes. Levels of
variation in this complex suggest that multiple species are involved.
Separating Central from South American forms would be the first step, supported
by the mitochondrial tree.
“C. No. There seem to be discordance in the
patterns. Winker lumped albivitta and
griseogularis, presumably because of
plumage similarities, but Puebla-Olivares et al (2008) found griseogularis more closely related to atrogularis and not reciprocally
monophyletic. Therefore, there is no obvious split within the Andes that is
supported by both plumage and genetics.”
Comments
from Areta:
“A complicated proposal that is made more difficult by the lack of
morphological and genetic data from key taxa and places. I am not so sure that
vocalizations are so constant in Aulacorhynchus.
More careful analyses may come up with solid differences even among taxa
considered to be vocally similar.
“A.
YES to separating northern from southern species based on morphological and
genetic data.
“C.
NO. Although it is possible that more species can be recognized within the
southern species, more information is needed on how all the taxa sort out
phylogenetically and morphologically before deciding on how to split them. For
example, griseigularis may be a good species,
resembling albivitta but more closely related
to atrogularis (this is what one would expect
for two good yet unrelated species, as highlighted by Van). Also, including griseigularis in albivitta
as proposed by Winker 2016 creates a paraphyletic albivitta
(see Figure 2 in Puebla-Olivares et al 2008). The two individuals
phenotypically like albivitta that have mtDNA
haplotypes more closely related to atrogularis/cyanolaemus/dimidiatus
(Ecuador Northeast 1 and 2; an important result that was not
discussed by Puebla-Olivares et al 2008, but that was put in perspective by
Winker 2016) add more mud to the question, blur the independence of atrogularis/cyanolaemus/dimidiatus and albivitta,
and indicate the need of further sampling to understand what is going on there.
Likewise, the lack of samples of lautus and phaeolaemus precludes taking any fully informed
decision. It looks like we are close to reaching a point in which all taxa will
be sampled, until then, some key details suggest that recognizing additional
species with present data is unsatisfactory.”
Comments
from Schulenberg:
“I can live with Northern and Southern
Emerald-Toucanet (so a Yes on 777B);
I'm more upset by the hyphen that I am by the modified compound group name.
“Regarding 777C, which I'm not voting on, comments mention that
"cyanolaemus and atrogularis hybridize freely or do
they?" Everyone goes on to assume that they do. The evidence on
hybridization is rather thin. Haffer (1974) based the evidence of hybridization
on six specimens from the southern distributional limit of cyanolaemus, all of which "because of their larger size and
blue throat ... are closer to cyanolaemus
but display a remarkably intermediate bill color". I'm struck that of
these six specimens, from two localities, not a single one shows the slightest
evidence on introgression in either plumage or size. I don't know what would
contribute to the intermediacy of the bill color, but given the lack of
divergence from cyanolaemus in
anything but bill color, I think you'd have to consider other possibilities
here. Furthermore, as stressed to me by Dan Lane (who I'm surprised hasn't
spoken up yet about this), it's entirely possible that cyanolaemus and atrogularis
are not even in contact. Aside from the north/south replacement, these two also
occur at different elevations: cyanolaemus
is a typical montane emerald toucanet, which in Peru occurs above 1600 m, or
but atrogularis is in the lowlands,
from a few hundred meters up to 1000-1200 m or so. In other words, in the
region where cyanolaemus and atrogularis might overlap, you'd expect Aulacorhynchus derbianus to occur
between them in elevation.
“Back to names In the event that SACC were to recognize atrogularis, or cyanolaemus + atrogularis,
as a species, then the name "Southern" applied to the albivitta group would be ridiculous: A. albivitta would be more southern than
Northern Emerald-Toucanet, A. but it would not be the southernmost species of
emerald toucanet, since A. atrogularis
would occur south of Southern Emerald-Toucanet. I'm not going to submit a vote
on this, however, until it's relevant, and I know what taxa would be included
in the constituent species. Should there be further splits in this complex, I'd
prefer to ditch the "emerald toucanet" part of the name entirely, and
use novel names for all constituent parts.”
Comments from Bonaccorso:
“A YES. According to Puebla et al. (2008) there is enough
phylogenetic evidence to separate both clades. As Remsen mentioned (and I know
I don´t vote on common names), we should not call them Northern and Southern
Emerald Toucanets. Biogeographically A. p. cognatus is not “Northern”
but inhabits the Darien´s mountains, which are technically in South America. I
think this decision may be minimally modified in the future if we find out that
A. p. cognatus is more related to the South American clade, as would be
expected. In Puebla et al. (2008) we only had one sample of A. p. cognatus,
and analyses were only based on mitochondrial DNA. If A. p. cognatus was
more related to the South American clade, then the decision of separating these
two major clades would be much easier.
“C. NO, for now; contra Puebla et al. 2008) and Bonaccorso et al.
(2011). From the phylogenetic perspective, many forms are not well represented
or not represented at all in our previous work, and mitochondrial DNA may not
be the best marker if at least some hybridization-introgression is going on.
“These are some of my thoughts:
1) It does
not make much biogeographic sense that A. p. griseigularis (from the
western slope of the Central Andes of Colombia) is more closely related to A.
p. atrogularis, unless extinction happened in the intervening area (which
seems implausible). Such a phylogenetic pattern could arise if genes from A.
griseigularis are leaking to A. p. atrogularis through a more
continuous A. griseigularis-albivitta-cyanolaemus-atrogularis “axis”.
More sampling (both genetic and morphologic) is needed along eastern Ecuador to
understand potential contact zones much better. This is not easy because
“shotgun-based” collecting in Ecuador is practically impossible.
2) As Nacho
mentioned, the two samples from northeast Ecuador (Ecuador Northeast 1, and 2;
Fig 2. in Puebla et al. 2008), should be A. p. albivitta, based on
geographic distribution. The position of these samples in the tree make A.
p. albivitta paraphyletic. This “misplacing” may be another example of gene
introgression.
3) As
mentioned before, the possibility of limited gene flow in contact zones should
not be enough evidence for discarding otherwise good biological species. Still,
more data is needed to understand the magnitude of hybridization-introgression
(if any) in these areas.”
Comments from Lane:
“A. YES, in part to be
in line with NACC classification, and in part because the Bonaccorso et al tree
does seem to show a deep branch for this split.
“B. NO. I find
"Northern E-T" type names incredibly ugly, and I think we can do
better than this. Just a quick stab on my part would result in "Middle
American Toucanet" and "Andean Toucanet" for starters.
"Variable Toucanet" would also work for the South American species.
“C. I find it difficult
to agree to this with so little firm evidence of how many lineages we're
dealing with here. As Tom alluded in his comments above, I am not convinced
that cyanolaemus and atrogularis actually come into contact today
and thus that "intermediate" birds are in fact actual intermediates.
A study needs to be performed to confirm this supposition, and I suspect it
will find hybridization (if there ever was any) to be ancient. More
importantly, I believe there ARE vocal differences between cyanolaemus
and atrogularis/dimidiatus, but this is anecdotal at best (and I
can't speak with respect to more northerly populations in South America). In
the end, the lack of a well-sampled phylogeny of this group and the poor
understanding of contact zones among its members leads me to say that, for now,
perhaps best to leave all South American birds as one thing until we have a
better set of facts to act upon.
“D. Well, I voted no
for C, so NO here too.. but even if I thought yes for C, I would still think
these choices of names are not suitable.”
Comments from Zimmer:
“A. YES, tentatively,
primarily because the branch lengths for this split (in the tree from
Bonaccorso et al) do appear to be deep, but also, knowing that NACC has already
accepted this split. I do have some
issues with this two-way split however, particularly as they relate to the
“nearest-approach neighbors” in Panama, A.
[c.] caeruleogularis and A. [c.]
cognatus, which Winker proposes as constituting a 3rd
Middle/Central American species (= “Blue-throated Toucanet”). Looking at the situation from the perspective
of biogeography, the typical pattern of taxon-replacement that we see in
Panama, is for Talamanca-Chiriquí highland birds (in this case, caeruleogularis) to drop out in the
isolated mountains of central Panama (e.g. Coclé-Panama provincial border
region), with lowland birds extending eastward at least to the Bayano River valley before being replaced in the lowlands
of Darién by taxa typical of the Chocó region of Colombia & NW Ecuador, and
with taxa occupying the Darién highlands being either endemic to that region,
or, showing affinities to Andean taxa in Colombia. In the case of these toucanets, one form or
another occupies (in broken fashion) the foothills and highlands right across
the country, from the Chiriquí highlands (definitely caeruleogularis) in the W to the Darién highlands (definitely cognatus) in the E, including the
Coclé-Panama provincial border, W Comarca Kuna Yala
(formerly San Blas), and W Panama Province, all geographically intermediate
points, in which, according to HBW, the resident form should be cognatus. But, such a distribution does not fit the
biogeographic pattern of taxon replacement that I laid out above. I would expect that turnover in
reproductively isolated (biological) highland species, if was going to happen
anywhere in Panama & Costa Rica, would come first between the lowland gap
in Nicaragua/N Costa Rica and the Talamanca-Chiriquí highlands, and that if
there were additional species-replacements, the next break would be in the
Darién highlands. So, to me, the
3-species (prasinus, wagleri,
caeruleogularis) for Middle/Central America treatment advocated by Winker
makes more sense than does a straight-up two-way Middle/Central versus South
American split. As Elisa noted in her
comments, cognatus has yet to be
adequately sampled genetically, so we don’t yet know where its affinities
lie. It’s important that such samples
should come from across the purported range of cognatus, and not just from C Panama, because I suspect that Darién
birds are more closely allied to the South American clade as Elisa postulated,
whereas it would make more sense for the birds from C Panama to belong with caeruleogularis.
B. “YES, for now. If 777A passes, and we are left with a
two-way, Central American versus South American split, then these proposed
names are the safest placeholders. Dan’s
suggestions of “Middle American” and either “Andean” or “Variable” are more
streamlined and appealing, but I think it would be premature to adopt either
until we find out more about the affinities of caeruleogularis and cognatus,
which could, depending on how things turn out, render “Andean” or “South
American” inappropriate for albivitta,
and “Middle American” as not exclusive to prasinus
(in the event that caeruleogularis is
shown to be worthy of splitting too).
“Northern” and “Southern” aside from being boring and “ugly”, have the
advantage of remaining accurate regardless of how the dust settles in Panama.
C. “NO. Even though the phenotypic differentiation is
highly correlated with genetic distance, and the only clear evidence of recent
hybridization is limited to the genetically closest pair of taxa, I just don’t
think we know enough about what is actually happening in potential contact
zones (including the true potential for contact between dispersing and resident
forms), vocal variation, and the importance of vocal differences or lack
thereof as prezygotic RIMs. Van’s points
about plumage and bill color differences not acting as effective RIMs in other
Ramphastid genera, and that vocal distinctions seem to be the most important
RIMs between other species of Aulacorhynchus,
both give me pause to placing too much stock in the obvious phenotypic
distinctions within the prasinus (sensu lato) group, particularly when, as
Santiago notes, there is apparent discordance between the phenotypic and
genetic patterns.
D. “NO. If 777C passes, “Southern” makes no sense for
albivitta, given that its range would
lie to the north of that of atrogularis. Also, “Black-throated” would only be accurate
when referring to nominate atrogularis,
since cyanolaemus, is
blue-throated. I think we need to wait
and see if 777C passes, and if it does, then come up with a separate proposal
to deal with English names.”
Additional
comments from Remsen:
“B.
YES. My NACC comments on this were based on a looming 3-way split. However, with this proposal passing only part
A, then I think Northern Emerald-Toucanet and Southern Emerald-Toucanet are a
good way to start. NACC has already used
the former. Although uninspired, and
using the group names that few like, I actually favor them in this case because
they make the sister relationship unambiguous with Aulacorhynchus and
because I like retaining “Emerald” – that’s a memorable name and connects both
species to the past, as both have been known as Emerald Toucanets “forever.”
“That
said, I see the merit in Dan’s comments and might vote that way if a follow-up
proposal is made. For now, sticking with
Southern just because NACC went with Northern.”
Comments
from Stiles:
“My tallies (trying to navigate the mixed comments on taxonomic changes vs.
E-names): A (the 2-species split)- 8 or 9 YES, 0-1 NO; therefore, this one
passes. B (N and S Em-Toucanets): 4-5 YES, 2-3 NO: does not pass; however, no
alternatives seemed to gain much acceptance either, so if 1-2 NO-voters or
non-voters change to YES, (with the understanding that this might be a
temporary solution pending filling in some of the numerous gaps in the data),
we can at least put this one to bed for now. C (split the S group into 2
species: 1YES, 8 NO, so this one fails and makes D irrelevant.”