Proposal (802) to South American
Classification Committee
Revise
familial limits and the linear sequence of families within the nine-primaried
oscines
Note from Remsen: This proposal passed NACC in 2017 and was implemented in
the 58th NACC supplement (Chesser et al. 2017, Auk. For NACC members’
comments on the proposal, which were 8-1 in favor, go to 2017-B6).
It is posted here with permission from Nick Mason and Kevin Burns; I have
marked extralimital taxa).
Effect on NACC: If approved, this proposal would reassign six
warbler species to different families, resurrect some previously used families,
and recognize three new families of nine-primaried oscines. Pending acceptance
of those familial classifications, this proposal would also change the linear
sequence of nine-primaried oscine families to reflect our growing knowledge of
their evolutionary relationships.
Background: The nine-primaried oscines comprise a
widespread, diverse assemblage of songbirds that accounts for nearly 10% of all
birds. In our current taxonomic classification, numerous taxa are included in
the linear sequence as incertae sedis,
which reflects our uncertainty of the phylogenetic placement of these taxa
within the avian tree of life. In addition, six species assigned to Parulidae (Zeledonia coronata, Icteria virens, Xenoligea
montana, Microligea palustris, Teretistris fernandinae, and Teretistris fornsi) do not show a close
relationship to wood warblers and can now be assigned elsewhere. A recent
series of publications has improved our understanding of evolutionary
relationships within and among lineages of nine-primaried oscines, providing an
opportunity to improve our current classification.
New Information: Barker et al. (2013) conducted a multilocus
systematic study of nine-primaried oscines in which they sampled exemplars from
every genus of Cardinalidae, Emberizidae, Icteridae, Parulidae, and Thraupidae.
The study also sampled multiple exemplars of genera known to lack monophyly and
genera not recognized by the current taxonomy for a total of 204 ingroup taxa.
Barker et al. (2013) also included representatives of Fringillidae and
Motacillidae as outgroup samples for a total of 213 taxa in the complete data set.
The study included two mitochondrial gene regions (ND2 and cyt b) in addition to one exon (RAG1) and
three introns (MB-I2; FGB-I5; sex-linked ACO1-I9). The authors conducted
multiple phylogenetic analyses on different partitions of the data set, including
analyses on each gene region separately, a concatenated analysis, and a species
tree analysis (Fig 1). The authors also conducted time-calibrated phylogenetic
analyses and considered associations between the stem age of families and their
species richness (Fig 2). A later study (Barker et al. 2015) combined these
data with mitochondrial data from additional species resulting in a total data
set of 791 of an estimated 832 species involved in this large clade (95%
sampling). Barker et al. (2015) used the concatenated and species tree
phylogenies of Barker et. al (2013) to create a “pseudoposterior” distribution
of species-level supertrees. In this proposal, we focus our discussion on the
relationships presented by Barker et al. (2013) to propose a new familial
classification for the nine-primaried oscines. In proposing a new familial
classification, we try to minimize changes to the current classification unless
strongly supported nodes imply that the current classification is inaccurate.
We consider support for recognizing each family in our proposed classification
and present them below in a revised linear sequence (see also Table 1). Type
designations and diagnoses for all new families are given in the Appendix of
Barker et al. (2013), and these families are already in use by Winkler et al.
(2015) and Lovette and Fitzpatrick (2016).
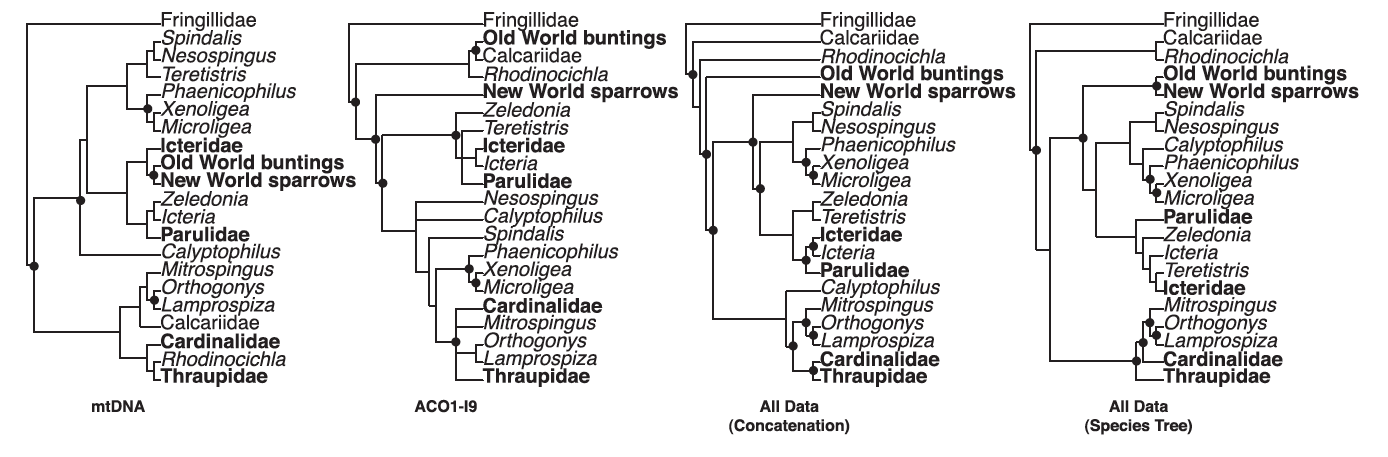
Figure 1: Phylogenetic analyses
of Barker et al. (2013) regarding relationships among nine-primaried oscines.
Phylogenies presented here are based on mtDNA, sex-linked ACO1-19, concatenated data sets, and species tree analyses.
Dark circles indicate strongly supported nodes.
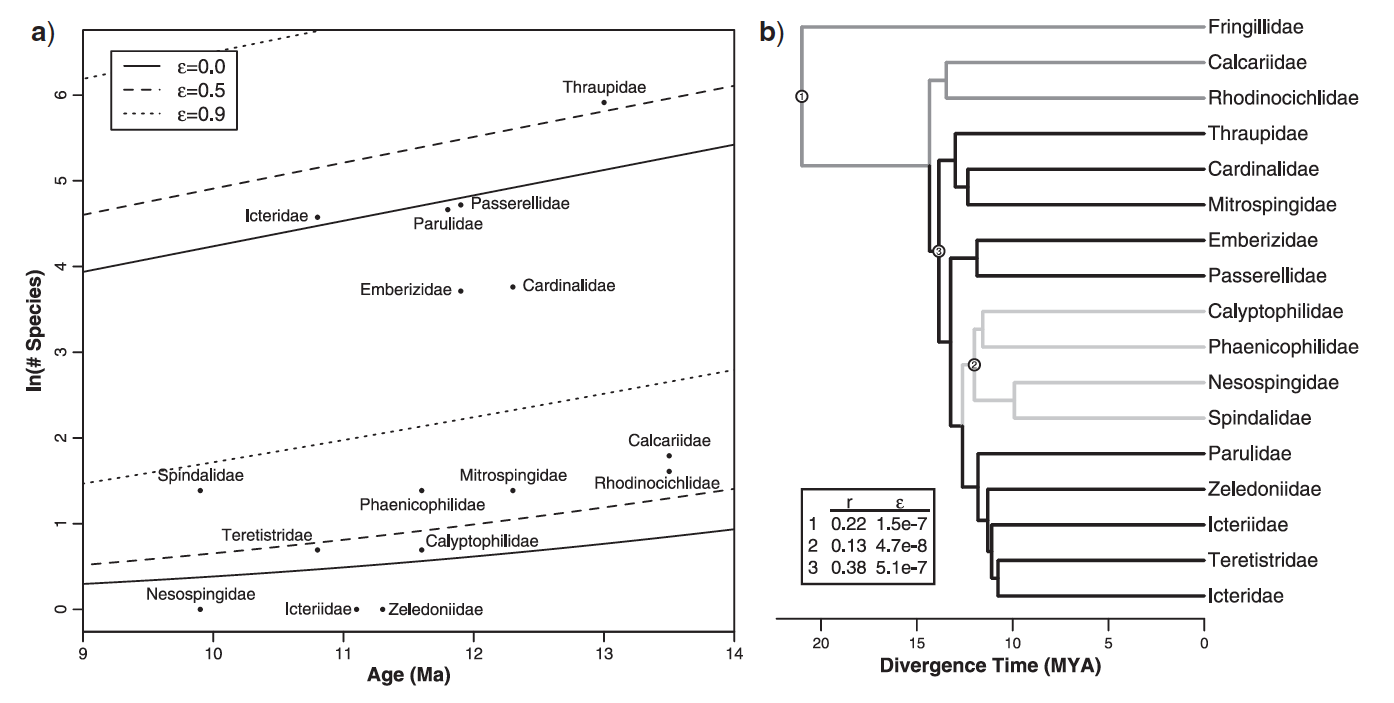
Figure 2: Panel (a) shows
associations between species richness and stem age for proposed families. Panel
(b) shows a time-calibrated phylogeny of lineages within the nine-primaried
oscines.
Fringillidae — This long-recognized
family of finches forms a monophyletic group that is sister to the remaining
nine-primaried oscines. This phylogenetic placement was well supported in all
analyses presented by Barker et al. (2013). No changes are needed to this family.
Calcariidae (extralimital) — Longspurs and snow buntings, including
six species in three genera. No changes to the composition of this family are
required by new studies. In addition, we propose retaining this family in the
linear sequence following Fringillidae. The placement of this family varied
among different analyses presented in Barker et al. (2013). In the concatenated
data set, it was sister to the remaining nine-primaried oscines; in the species
tree analysis, however, it was inferred to be sister to Rhodinocichla with low support, and these two taxa were sister to
the remaining nine-primaried oscines. The placement of Calcariidae varied
substantially among gene trees, albeit typically with low support. An exception
was the gene tree for ACO1-I9, in which Calcariidae was sister to the Old-World buntings (Emberizidae) with high
support. Nonetheless, the topologies inferred with the concatenated and species
tree analyses were largely congruent in placing Calcariidae near the beginning
of the sequence, and retaining Calcariidae as a family near the beginning of
the sequence will cause the least disruption to the current classification.
Rhodinocichlidae — Barker et al. (2013)
proposed placing Rhodinocichla rosea,
the Rosy Thrush-Tanager, in its own family, Rhodinocichlidae. Historically,
this aberrant species has been difficult to place, with some authors noting
similarities to Mimidae. Eisenmann (1962) showed that it lacked similarities to
Mimidae, but shared features with nine-primaried oscines. He considered it best
placed with the tanagers, acknowledging that this placement was in part due to
the varied nature of tanagers themselves. Most classifications have considered
this species to be a tanager (e.g., AOU 1998). With limited genetic data, Seutin
and Bermingham (1997) were able to confirm a closer relation of Rhodinocichla to nine-primaried oscines
than to Mimidae. However, they were unable to definitively show that it was a
tanager. In the current AOU classification, Rhodinocichla
is listed as incertae sedis.
Barker et al. (2013) reaffirmed the genetic distinctiveness of this species,
with no clear relationship to existing families. Rhodinocichla was sister to the remaining nine-primaried oscines in
the concatenated analyses, while the species tree analyses inferred a sister
relationship between Rhodinocichla and
Calcariidae (Fig. 1). Neither relationship was strongly supported, however.
Nonetheless, it seems likely that Rhodinocichla
falls outside of the clade that includes the most recent common ancestor of
Old World buntings and tanagers. Rhodinocichla
is also phenotypically and behaviorally distinct: it has vinaceous plumage
on the throat and belly and forages among leaf litter in the undergrowth. We
therefore suggest that Rhodinocichla be
placed in the previously recognized monotypic family Rhodinocichlidae. Note also the relative older stem age of Rhodinocichla, compared to other
recognized nine-primaried oscine families (Fig. 2a).
Emberizidae — In the current AOU classification, Emberizidae
includes both Old World buntings and New World sparrows. In the phylogenies
presented by Barker et al. (2013), Old World buntings were sister to New World
sparrows in the species tree analysis, but sister to the remaining
nine-primaried oscines in the concatenated data set (Fig. 1). These alternate
topologies were strongly supported in their respective analyses. Thus, there
was significant conflict in the placement of these two groups relative to each
other. However, Old World buntings and New World sparrows consistently formed
two monophyletic groups that were mutually exclusive. Barker et al. (2013)
argued for splitting these two groups into separate families for the sake of
future taxonomic stability and to recognize biological and biogeographic differences
between the two clades. In addition, Calcariidae is sometimes seen as being
more closely aligned to Old World Buntings. Using three separate names:
Calcariidae, Emberizidae (for Old World buntings only), and Passerellidae (for
New World sparrows, see below) could prevent future taxonomic problems in case
new data support different relationships among these three clades. Emberiza, Latoucheornis, Melophus, and Miliaria were assigned by Barker et al. (2013) to Emberizidae, but
only Emberiza occurs in the AOU area.
Passerellidae — As discussed above, the New World sparrows
are currently considered part of Emberizidae by the current AOU classification.
Species tree analyses suggested that they are sister to the Old World buntings,
while the concatenated data indicated that they are sister to the lineage
containing the most recent common ancestor of Spindalis and Parulidae (Barker et al. 2013; Fig. 1). Despite
uncertainty in its topological placement, Passerellidae consistently formed a
strongly supported, monophyletic group that excludes other taxa. For the
reasons outlined above, we recommend reassigning all genera in our current
Emberizidae (except Emberiza) to
Passerellidae (the oldest family name available for this group; Barker et al
2013).
Calyptophilidae (extralimital) —
Calyptophilus consists of two species
of chat-tanagers endemic to Hispaniola and historically considered part of
Thraupidae. Calyptophilus is placed
as incertae sedis in the current AOU
classification. In the concatenated analysis, Calyptophilus was sister to a clade containing Mitrospingidae and
Thraupidae (Barker et al. 2013; Fig. 1). In the species tree analysis, Calyptophilus was sister to
Phaenicophilidae (Barker et al. 2013;
Fig. 1). Given that there are no genetic or morphological characters uniting Calyptophilus to other species, we
recommend following Barker et al. (2013) and resurrecting Calyptophilidae for
these species.
Phaenicophilidae (extralimital)
— This family would include species from three genera that share a
biogeographic affinity in the Caribbean: Phaenicophilus,
Microligea, and Xenoligea. Phaenicophilus was
traditionally included in Thraupidae, but is incertae sedis in the current AOU classification, whereas Microligea and Xenoligea are included in Parulidae. Genetic studies (e.g., Lovette and Bermingham
2002, Klein et al. 2004) have shown that Microligea
and Xenoligea are not closely related
to Parulidae, and Klein et al. (2004) identified a close relationship among the
three genera considered here, to the exclusion of warblers and tanagers. Barker
et al (2013) confirmed this result. In both the concatenated analysis and the
species tree analysis, these three genera formed a strongly supported clade
(Barker et al. 2013). Due to their common ancestry, plumage similarities, and
biogeography, we propose that these genera be classified together in a single
family. Phaenicophilidae was previously used for Phaenicophilus alone and can now be expanded to include Microligea and Xenoligea (Barker et al. 2013).
Nesospingidae (extralimital) —
Barker et al. (2013) proposed a new monotypic family for Nesospingus speculiferus, the Puerto Rican Tanager. Nesospingus has historically been
included in Thraupidae, but it is currently considered incertae sedis in the AOU classification because several studies
(including Barker et al. 2013) have shown this species to fall outside the
‘core’ Thraupidae. Instead, Barker et al. (2013) inferred a sister relationship
between Nesospingus and Spindalis in both the concatenated and
species tree analysis, although this node was not strongly supported. In
addition, the position of the Spindalis/Nesospingus
clade was not strongly supported in any analyses, although it typically
appeared in clades with other Caribbean genera.
Thus, the general placement of Nesospingus
within the broader clade that contains the most recent common ancestor of Nesospingus and Parulidae remains
uncertain. Following Barker et al. (2013), we propose using the family
Nesospingidae for this genus. Alternatively, an argument could be made to merge
Spindalis and Nesospingus into a single family based on their consistent
placement as sister taxa. This has the advantage of making the age of the clade
more in line with other families of nine-primaried oscines (Fig. 2a). However,
support is not strong for this relationship.
Spindalidae (extralimital) —
Barker et al. (2013) proposed a new family for Spindalis, a genus consisting of four non-migratory species endemic
to the Greater Antilles. The current AOU classification treats Spindalis as incertae sedis. As discussed in the previous section, Barker et al.
(2013) inferred a sister relationship between Spindalis and Nesospingus with
weak node support. Because the relationships of Spindalidae and Nesospingidae
remain uncertain, recognizing both taxa as families presents a stable solution
that improves on the current classification.
Zeledoniidae (extralimital) —
Barker et al. (2013) proposed resurrecting the monotypic family Zeledoniidae
for Zeledonia coronata, the
Wrenthrush. This species, endemic to Costa Rica and western Panama, has long
been recognized as morphologically and ecologically distinct (Hunt 1971). It
has previously been classified in Turdidae (e.g., Mayr and Amadon 1951, Beecher
1953) or in its own family, Zeledoniidae (e.g., Wetmore 1960). Raikow (1978)
analyzed myological characters and suggested that the species belonged to
Parulidae, and the species was placed in Parulidae in the 1998 AOU checklist.
However, comprehensive genetic analyses (starting with Lovette and Bermingham
2002) showed this species to fall well outside Parulidae, and it is currently
treated in the AOU classification as incertae
sedis. In the Barker et al. (2013) trees, the placement of Zeledonia within the nine-primaried
oscines was uncertain, as it differed
between the concatenated and species tree analysis with low node support in
both phylogenies (Fig. 1). Given that morphological, ecological, and genetic
data have failed to find a strong connection of Zeledonia to any other nine-primaried oscine, we argue that it is
time to return this species to the monotypic family Zeledoniidae.
Teretistridae (extralimital) —
Teretistris has historically been
included with Parulidae, but comprehensive genetic studies (starting with
Lovette and Bermingham 2002) showed that it falls outside Parulidae. The two
species in the genus are still placed in Parulidae in the current AOU
classification. Barker et al. (2013) proposed using the previously recognized
family, Teretistridae, which would include Teretistris
fernandinae and Teretistris fornsi.
The placement of Teretistris was
uncertain among the nine-primaried oscines; it was inferred as sister to Zeledonia in the concatenated analysis
and sister to Icteridae in the species tree analysis, although neither
placement received strong support (Fig. 1). Thus, we recommend following Barker
et al. (2013) and recognizing family Teretistridae.
Icteriidae (extralimital) —
Barker et al. (2013) proposed resurrecting Icteriidae for Icteria virens, the Yellow-breasted Chat. This species has
traditionally been classified with Parulidae, but that placement has long been
questioned (taxonomic history summarized in Lovette and Bermingham 2002).
Genetic data in Lovette and Bermingham (2002) showed that the species is not
part of the ‘core’ Parulidae and that the species was perhaps sister to
Icteridae. Some of the trees of Barker et al. (2013) contained a similar
result, but others did not. In the concatenated analysis, Icteria was inferred as sister to Icteridae with strong support. In
the species tree analysis, however, Icteria
was placed as sister to a clade containing Teretistris
and Icteridae, although this did not receive strong support. Given the lack
of consistent support for this species’ placement, and given the general
distinctiveness of this species relative to warblers and to blackbirds, we
agree with Barker et al. (2013) and recommend removing Icteria from Parulidae and using Icteriidae for this species.
Icteridae — Barker et al. (2013) showed that blackbirds
and allies form a monophyletic group that has been long recognized as a family.
The placement of Icteridae remains uncertain—it was sister to Icteria in the concatenated analysis
with strong support, but has uncertain placement in the species tree analysis.
No changes in species composition of Icteridae are needed.
Parulidae — As mentioned above, six species that the AOU
currently classifies in Parulidae need to be assigned to other families in
order for the classification to be consistent with the data. The remaining wood
warblers formed a monophyletic group in the trees of Barker et al. (2013). In
the concatenated data set, Barker et al. (2013) inferred with strong support
that Parulidae is sister to a clade containing Icteria and Icteridae. In the species tree analysis, it was sister
to a lineage that includes Zeledonia,
Icteria, Teretistris, and Icteridae, although this relationship was not
strongly supported (Fig. 1).
Mitrospingidae — Barker et al. (2013) proposed a new family
that includes Mitrospingus, Orthogonys, and Lamprospiza. These three genera formed a strongly supported clade
in both the concatenated and species tree analyses (Barker et al. 2013; Fig.
1). All of these genera have been
historically classified in Thraupidae, but none were sister to or nested within
Thraupidae in any of the phylogenies presented by Barker et al. (2013). Thus,
we recommend following Barker et al. (2013) to recognize Mitrospingidae for
these species.
Cardinalidae — Cardinals and allies have long been
recognized as a family, Cardinalidae. In Barker et al. (2013), they formed a
monophyletic group that is sister to Thraupidae in the concatenated data set
and sister to Mitrospingidae in the species tree analysis. No changes in
species composition are needed for this group.
Thraupidae — The tanagers and allies are currently
classified in the family Thraupidae. Barker et al. (2013) inferred a sister
relationship between Thraupidae and Cardinalidae in the concatenated data set.
In the species tree analysis, Thraupidae was sister to a clade containing
Cardinalidae and Mitrospingidae. No changes in species composition are needed
for this group; the committee dealt with these in a recent supplement.
Table 1: Current linear
classification is shown in the first column, while the linear classification
presented in this proposal is shown in the second column. Gray-shaded families are extralimital.
|
Current
Linear Classification |
Proposed
Linear Classification |
|
Fringillidae |
Fringillidae |
|
Calcariidae |
Calcariidae |
|
Parulidae* |
Rhodinocichlidae |
|
Thraupidae |
Emberizidae
|
|
Nesospingus (incertae sedis) |
Passerellidae |
|
Phaenicophilus (incertae sedis) |
Calyptophilidae |
|
Calyptophilus (incertae sedis) |
Phaenicophilidae |
|
Rhodinocichla
(incertae sedis) |
Nesospingidae |
|
Mitrospingus
(incertae sedis) |
Spindalidae |
|
Spindalis (incertae sedis) |
Zeledoniidae |
|
Emberizidae** |
Teretistridae |
|
Cardinalidae |
Icteriidae |
|
Icteridae |
Icteridae |
|
|
Parulidae |
|
|
Mitrospingidae |
|
|
Cardinalidae |
|
|
Thraupidae |
*
Includes Zeledonia, Teretistris, Icteria, Microligea, and Xenoligea
**
Includes Passerellidae
Recommendation: We recommend that the committee rearrange
familial limits within the nine-primaried oscines to reflect the findings of
recent molecular systematics studies, and modify the linear sequence of taxa
within the nine-primaried oscines to correspond to this new classification.
Although some may argue that this results in too many families, keep in mind
that this clade contains nearly 10% of all birds. Previous assignments were
largely based on the presumed importance of feeding morphology, but we now have
the opportunity to organize the diversity of this major group of birds using
phylogenetic evidence for the first time.
A
YES vote would accept the above classification.
Literature Cited:
AOU. (1998). Check-list
of North American birds, 7th ed. American Ornithologists’ Union,
Washington, DC.
Barker, F. K., Burns,
K. J., Klicka, J., Lanyon, S. M., & Lovette, I. J. (2013). Going to
extremes: contrasting rates of diversification in a recent radiation of New
World passerine birds. Systematic Biology, 62(2), 298–320.
http://doi.org/10.1093/sysbio/sys094
Barker, F. K., Burns,
K. J., Klicka, J., Lanyon, S. M., & Lovette, I. J. (2015). New insights
into New World biogeography: An integrated view from the phylogeny of
blackbirds, cardinals, sparrows, tanagers, warblers, and allies. Auk, 132(2),
333–348. http://doi.org/10.1642/AUK-14-110.1.s3
Beecher,
W. J. (1953). A phylogeny of the oscines. Auk, 70, 270–333.
Eisenmann, E. (1962).
On the systematic position of Rhodinocichla
rosea. Auk, 79, 640–648.
Hunt,
J. H. (1971). A field study of the Wrenthrush, Zeledonia coronata. Auk, 88, 1–20.
Klein, N., Burns, K.
J., Hackett, S. J., & Griffiths, C. (2004). Molecular phylogenetic
relationships among the Wood Warblers (Parulidae) and historical biogeography
in the Caribbean Basin. Journal of
Caribbean Ornithology, 17, 3–19.
Lovette, I. J., &
Bermingham, E. (2002). What is a wood-warbler? Molecular characterization of a
monophyletic Parulidae. Auk, 119(3), 695–714.
Lovette, I. J., &
J. W. Fitzpatrick, eds. (2016). Handbook of Bird Biology. Third Edition.
Wiley-Blackwell, Hoboken, New Jersey.
Mayr, E., & Amadon,
D. (1951). A classification of recent birds. Amer. Mus. Nov., 1496, 1–41.
Raikow, R. J. (1978).
Appendicular myology and relationships of the New World nine-primaried oscines
(Aves: Passeriformes). Bull. Carnegie Mus. Nat. Hist. 7,
1–43.
Seutin, G., &
Bermingham, E. (1997). Rhodinocichla
rosea is an emberizid (Aves; Passeriformes) based on mitochondrial DNA
analyses. Molecular Phylogenetics and Evolution, 8(2), 260–274.
http://doi.org/10.1006/mpev.1997.0426
Wetmore, A. (1960). A
classification for the birds of the world. Smithson. Miscell.
Collect. 139, 1–37.
Winkler, D. W.,
Billerman, S. M., & Lovette, I. J. (2015). Bird Families of the World. Lynx
Edicions, Barcelona, Spain.
Names and Affiliations of Submitters: Nicholas A. Mason,
Cornell University, and Kevin J. Burns, San Diego State University
Comments from Stiles: “YES. Overdue for SACC action! Given the wealth of
genetic data, I see no reason to delay, especially as NACC has implemented
these.”
Comments from Robbins: “YES. The genetic data and the reasoning by
Barker et al. are solid, so I vote Yes for making the proposed linear
arrangement.”
Comments from Claramunt: “YES. Important update to the
family-level classification. It is
somewhat inconvenient that the classification resulted in multiple new families
with few species, but that’s what the phylogenetic structure indicates so far
(and most are extralimital to SACC anyway).
Among them, I can’t resist to comment on how inconvenient it is to
create the family Icteriidae due to obvious similarity with Icteridae. It will be a constant source of confusion and
errors and another reason for not-taxonomists to hate taxonomy. I would have waited for new evidence to
really nail down the phylogenetic position of Icteria before erecting a new family in this case.”
Comments from Pacheco: “YES. I find only good reasons to let go of this
reorganization already implemented by the NACC.”
Comments
from Zimmer:
“ “YES. The genetic data and the
rationale for the proposed changes all seem solid, and they all make more
biological sense to me based on what I know of the birds involved in life, than
does any former/current arrangement.”