Proposal (945) to South
American Classification Committee
Split Piranga flava complex into multiple species
Effect on SACC
classification: This would split
our Piranga flava into two or more species.
Background: This is a well-known problem in species limits
that has been dealt with differently by different authors for at least 120
years. Our current note reads as
follows:
Zimmer (1929)
was the first to treat all members of the P. flava group as a single
species, and this has been followed by most subsequent authors, although AOU
(1983, 1998) recognized three subspecies groups, and Isler & Isler (1987)
suggested that each might be better treated as separate species. Meyer de Schauensee (1966) and Ridgely &
Tudor (1989) also proposed that this species probably consists of two or three
separate species. Two of these occur in
South America: nominate flava of southern and eastern South America, and
the lutea group of the Andes region (and also Panama and Costa
Rica). See Zimmer (1929) concerning
earlier claims of sympatry between flava and lutea. Burns (1998) proposed that the three
subspecies groups should be treated as three phylogenetic species, and possibly
biological species, based on comparative genetic distance data within Piranga. Ridgely & Greenfield (2001) and Hilty
(2011) treated the
three groups as separate species. Haverschmidt and Mees (1994) treated the
subspecies haemalea of the Tepuis as a separate species from P. flava
based on habitat differences. Manthey
et al. (2016) found that haemalea was not part of the lutea
group. SACC
proposal needed.
Ridgway (1902) treated
the complex as 2 species, as follows:
1. Piranga
hepatica (Hepatic Tanager): SW USA to Guatemala
2. Piranga
testacea: (Brick-red Tanager: Nicaragua to Bolivia (also with pine-lands
subspecies “Belize Tanager” P. t. figlina from Guatemala to Honduras)
Although many of the
Neotropical subspecies were not yet described, haemalea of the Tepui
region was described in 1883, but was not mentioned by Ridgway, nor was any
member of the even older lowland flava=azarae group of
south-central South America. This might
suggest that Ridgway did not consider them part of this group.
Zimmer’s (1929) 50+ page monograph on the group is the basis of our current
classification. However, this monograph
was written in an era when vocalizations were not taken into account – all
taxonomy was based on external morphology combined with distributional
considerations (e.g., sympatry/parapatry vs. allopatry). Here is Zimmer’s synopsis, and you can see
that similarities between the extreme northern and southern taxa strongly
influenced his reasoning for treating them all as conspecific:
“Examination
of numerous specimens of these forms and certain of their unquestioned allies
has fostered the belief that all are races of a single species whose
distribution extends from eastern Argentina to southwestern United States with
little interruption in continuity, though with lateral extensions into Brazil
and the Guianas and into Venezuela and the Guianas in two lines of development
which meet at their outward extremities. Throughout this extensive group, the
color, general pattern, size, shape of bill, and other major characters are
substantially identical or are subject to variability which largely overcomes
the individual differences. There are certain features of plumage and molt
which seem to be present in all the forms under consideration but which are
different from those of the other congeneric groups. The various forms replace
each other geographically in all parts of the range. Finally, at the northern
and southern extremities forms are produced which are strikingly alike in
racial characters that are not shared by the intervening subspecies.
These considerations together present a
volume of evidence that is more than circumstantial. There is no question that
all the forms under discussion are of common phylogenetic origin. Some of them
are more strongly differentiated than others, some distinctly intergrade with
adjacent forms, while others are separated from their nearest allies by so
slight a gap in proportion to the individual variation in that direction that
the relationship is not seriously impaired. In the following treatment,
therefore, I have considered as races of P. flava all the forms under
discussion”.
Zimmer’s study was perhaps the most thorough
study of a single widespread Neotropical bird of that era. So, this situation is about as different from
an unjustified Peters lump as you can get.
Subsequent classifications all followed Zimmer
on this, including Hellmayr, Storer in Peters, Sibley & Monroe, AOU,
Dickinson & Christidis, etc., except for those mentioned in the SACC note
above and the recent HBW/BLI classification.
Isler & Isler (1987)
summarized the qualitative differences in songs and calls for the three groups,
as well as the rather exceptional range of habitats for a single passerine
species (e.g. from arid pinyon-juniper scrub to edges of cloud-forest). The habitat differences were remarked upon
and noted as exceptional by Zimmer himself. Ridgely & Tudor (1989) proposed
that at least 2 and maybe 3 species were involved.
Here is the breakdown
from Isler & Isler (1987), with distribution refinements from Dickinson
& Christidis (2014). Because this is
a complex proposal, I suspect these details might come in handy
(A) hepatica
group (extralimital)
1.nominate hepatica:
highlands of SE California, Arizona, and w. New Mexico S in western Mexico
to Oaxaca
2. dextra:
highlands of e. New Mexico [presumably this species also in Las Animas Co., CO]
and SW Texas south on Caribbean slope to Chiapas
3. albifacies: in highlands of w. Guatemala to n. Nicaragua
4. figlina: lowland pine savannahs of
Belize and e. Guatemala
5. savannarum: lowland pine savannahs of extreme
e. Honduras and NE Nicaragua
(B) lutea
group
6. testacea: highlands of n. Costa
Rica to e. Panama (Darién)
7. faceta: Santa Marta to
highlands of Venezuela; Trinidad
8. haemalea: tepui region from
c. Venezuela, n. Brazil, c. Guyana, c. Suriname
9. toddi: two spots in n. Andes of Colombia
10. desidiosa: W. Andes of Colombia
(Antioquia to Cauca)
11. nominate lutea:
Andes from Nariño S through Ecuador and Peru to c. Bolivia (Cochabamba)
(C) flava
group
12. macconnelli:
lowlands of n. Brazil (Roraima) and the southern Guianas.
13. saira:
lowlands of e. Brazil from Amapá south (patchily) to Mato Grosso and Rio Grande
do Sul
14. rosacea:
lowlands of se. Bolivia e. Santa Cruz)
15. nominate flava:
foothills of E Bolivia from Cochabamba and w. Santa Cruz, S in lowlands to Paraguay, n. Argentina and
Uruguay [note that if the Bolivian records pertain to breeding birds, then this
is not strictly a lowland taxon; however, because this is an austral migrant, I
would not be surprised if these foothill records are wintering birds only –
this needs to be sorted out. ]
Here is the HBW plate
by H. Burn that illustrates 5 of the subspecies:

Burns (1998) proposed that the three subspecies group be treated as
separate phylogenetic and perhaps biological species based on comparative
genetic distance data (cyt-b); however, this was based on just seven specimens,
only one from the lutea group.
Burns concluded: “The DNA data of this study add to
the morphological, distributional, and ecological evidence that suggest that
the three subspecies groups of P. flava represent different
phylogenetic, if not biological species.”
New information
(since the original SACC classification): There really isn’t much in the way of new quantitative data.
Ridgely and
Greenfield (2001; Ecuador book) cited Burns (1998) for their treatment of Andean
lutea group as a separate species (Highland Hepatic-Tanager) from
lowland flava group (Lowland Hepatic-Tanager) as well as the northern hepatica
group (Northern Hepatic-Tanager), as did Hilty (2003; Birds of Venezuela) and
Restall et al. (2006; Birds of Northern South America. Vol. 1); however, note
that Burns was rightfully hesitant in calling them biological species.
Manthey et al. (2016) used Piranga to compare
different genetic techniques (UCEs vs. RAD-seq). They used only 6 individuals of the flava
complex, two from each subspecies group. Although taxon-sampling was weak, they
corroborated what had been concluded by Zimmer nearly a century earlier, i.e.
that the three groups formed a monophyletic unit. However, they found that lutea, was
paraphyletic: the Andean sample (N. Peru, ergo nominate lutea) was
sister to the two samples of hepatica (from El Salvador, ergo albifacies),
but the sample from Guyana (haemalea) was sister to the two samples of
lowland flava (one from Guyana, ergo presumably macconnelli and
one from e. Bolivia, ergo likely rosacea), both with strong support.
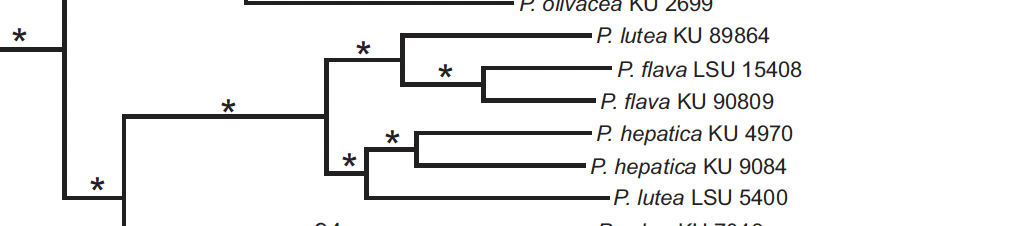
A problem here is that both KU samples from Guyana are
listed as from the same locality (“Upper Takutu”), which is a lowland savanna
locality (fide M. Robbins). For a
second, I thought I’d found evidence of sympatry of lutea and flava
groups, and thus automatic species rank for both.
But
because Manthey et al. didn’t point that out, I suspected an error in their
table. So I checked, and sure enough,
SACC’s Mark Robbins, who collected both specimens (and who obviously was not
asked to go over the paper), told me that this turns out to be an undetected copy-paste
error. From Mark:
“90809: 1.5 km S of Karaudanawa, in the upper Rupununi, i.e.,
lowland site; ID as macconnelli.
“89864: Acai Mts, in the extreme south [of Guyana]! ID as haemalea”
Nonetheless, it still remains that, as Manthey et al.
pointed out, the lutea group as currently constructed is not
monophyletic. The tepui subspecies haemalea
groups with the lowland flava group even though in plumage and
elevation, it fits with the Andean lutea group. Therefore, either the haemalea group
should be transferred to the flava group, which would not make sense
from plumage, or be treated as its own group.
Given that Haverschmidt and Mees (1994) treated haemalea as a
separate species, and that haemalea does not fit with flava in
plumage or elevation, this should be at least treated as a separate, fourth
group in analyses. Finally, that Robbins’
two specimens are only about 150 km from each other, but have such strong
genetic and phenotypic differences, the evidence for treating haemalea
as a separate species from flava, much less lutea, ironically could
be considered stronger than that for any of the other proposed splits even
though it’s never been recognized as a separate group.
Time for a mini-rant.
The above is yet another vivid demonstration of the importance of broad
taxon-sampling. In terms of genetic
sampling, one cannot just assume that a group of subspecies are a monophyletic
group unless all the taxa are sampled.
For all we know, the isolated lutea subspecies in the north group
with haemalea rather than nominate lutea, or form their own group,
or each forms a separate group, or testacea actually groups with
hepatica, or …. .
With respect to Manthey et al., with such limited
taxon-sampling (only 5 of 15 subspecies represented), I find it hard to extract
any firm evidence for species rank either way.
I personally don’t think the use of comparative branch lengths and
genetic distance can be used as a metric for species limits, but even so the
longest branch between the two clusters of Summer Tanager (P. rubra) samples
is longer than any between any of the Hepatic Tanager groups despite the enormously
greater geographic distances among samples of the latter.
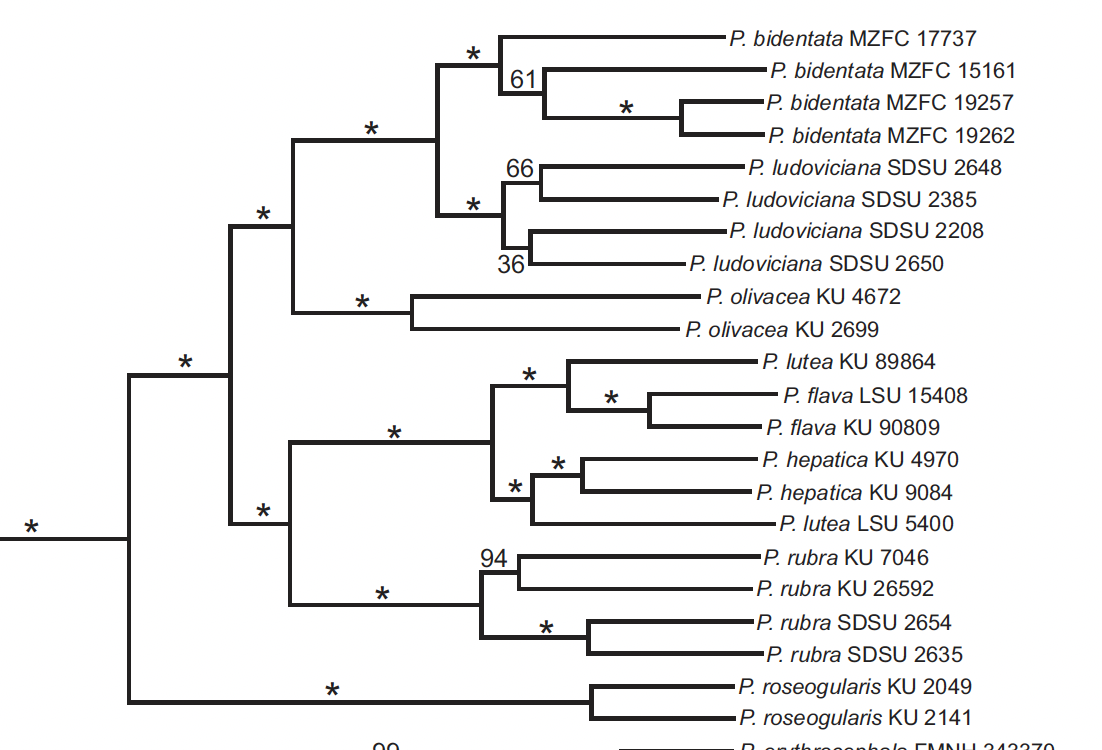
By the way, Manthey
et al. is cited as evidence for species rank by HBW/BLI and “IOC”.
As for vocalizations,
Boesman (2016) presented sonograms of songs and calls for each. The N for song sonograms was 3 for flava
group , 3 for lutea group, and 2 for flava group, with location/subspecies
not specified. The N for calls was 4 hepatica,
3 for lutea, and 5 for flava, also with location/subspecies not
specified except for one for testacea in the lutea group, which
appears to be the most different of all.
Here’s what my
impressions are from the sonograms. In
terms of song, I see more differences within the hepatica and lutea
groups as I do between them. As for flava,
the notes themselves do seem on average more complex, as noted by Boesman: “Song of flava group
seems to have the most complex-shaped notes, lacking any simpler-shaped notes.
Many notes are complex underslurred, and apparently there is little variation
in note shape (few different note shapes), unlike other races.” As for call notes,
those of hepatica and flava look very similar to me, but those of
flava look more complex, as noted by Boesman: “Call of flava group is
also clearly different, having an upslurred ending, while both other groups are
about identical, having a very sharp upturned V-shape.“
I suspect Peter Boesman would be the first to tell you
that this sort of sampling and qualitative comparisons has its problems. I will also repeat the same mini-rant that on
taxon-sampling that I did on the genetic data.
Boesman’s studies of this and many other groups are valuable for
pointing out potential issues (note his strange recording of testacea
mentioned above) and can serve as launching pads for more thorough
studies. But until all the taxa are
sampled, with sufficient N and careful attention to homology, their use as
determinants of species limits is perilous.
I think we should be grateful that he has set the table for the more
detailed analyses needed to really sort things out. Lots of potential here, but also lots of
sampling gaps in terms of subspecies and geography. One problem that could be fixed quickly is
that the location and subspecies need to be given for each recording; for
example, three lutea songs are represented, each looking fairly
different. But “which” lutea? They could be from testacea from Costa
Rica, nominate lutea from Bolivia, or any of the other four subspecies
in the group, including even haemalea from the tepui region, which we
now know is not a member of the group.
Or they could all three be from different individuals within any one of
the 6 subspecies.
Just to give you an
idea what the call notes are like, I grabbed a few links:
• A call note from
nominate hepatica from AZ (Richard Webster): https://xeno-canto.org/678668
• A call note from testacea
from Panama (Peter Boesman): https://xeno-canto.org/271527
• a call note from
nominate lutea from Peru (Fabrice Schmitt): https://xeno-canto.org/102734
• A call note from
nominate flava from Paraguay (Fabrice Schmitt): https://xeno-canto.org/616311
• A call note from
haemalea from Guyana (Ted Parker): https://search.macaulaylibrary.org/catalog?taxonCode=heptan&tag=call®ionCode=GY
What stands out to me
from superficial browsing of these and others on xeno-canto is (1) as Boesman
(2016) noted about testacea, that twittered, crossbill-like call seems
to be standard, and if that is the case, then testacea has by far the
most distinctive call; (2) hepatica, lutea, and haemalea
calls sound fairly similar; (3) flava calls are higher-pitched and
slightly inflected, but in the background of the Paraguay recording, I think I
hear another bird giving a lower-pitched call more like northern birds. Obviously, a quantitative analysis of all of
the is needed, with larger N of presumed homologous calls. My gut impression is that when all the data
are analyzed, they will support testacea as a separate species, with the
obvious caveat that this is just from a brief perusal of what is easily
available.
Piranga songs are fairly
complex, and so the final analysis will not be easy. Note that the vocal pattern does not fit the
plumage pattern, e.g. lutea and hepatica are evidently the most
similar vocally, whereas flava and hepatica are most similar
plumage-wise (which is at the core of Zimmer’s rational for conspecificity
Discussion and
Recommendation:
This issue is a real
problem. On the one hand, I share the instinctive
feeling of just about everyone who has commented on the group post-Zimmer that
more than one species is involved, but I would bump it up to at least 5
potential species If this really is to
be treated as one biological species, then the breadth of habitat types covered
would be exceptional, ranging from arid, rocky, pinyon-juniper slopes in the
north to pine savanna to the margins of cloud forest and semi-humid montane
forest to tropical dry forest; the only common denominator might be that the
structure of the habitat is open woodland and edge. (House Wren and Squirrel Cuckoo are the only species
of woodland birds that I can think of offhand that could compete with Hepatic Tanager
for habitat breadth.) But it’s hard to
find conclusive evidence for treating them as separate species. The plumages differ, but not outside the
range of variation for many polytypic species.
The genetic data are too weak to be interpreted either way, especially with
the sampling gaps. The vocal
differences, even qualitatively, between the flava group vs. the other
two suggest multiple separate species, but are the data officially strong
enough for a split?
This complex is
begging for dissertation-level research.
Geographic areas in need of careful sampling are:
(1) the potential
contact areas between haemalea of the tepuis and macconnelli of
the lowlands. They come pretty close in
Guyana and Suriname, but are likely separated by unsuitable habitat: tall tropical
forest. If there is no sign of gene
flow, then that’s all you need to argue for species rank in my opinion, even if
not precisely parapatric.
(2) central Bolivia,
where it is unclear how close lutea and breeding flava come to
each other. The sampling in our Bolivia
book (Herzog et al. 2016) shows a continuous distribution from the Andes of La
Paz and Cochabamba through the foothills of Santa Cruz and Chuquisaca to the
lowlands of Santa Cruz and Tarija. If
these refer to resident populations, then there is a contact zone somewhere in
there, as Hellmayr (1929) noted
Specimens from w. Santa Cruz and Chuquisaca are all assigned to flava
despite their montane distribution. But
we also noted that flava is a partial austral migrant. A first pass through the dates, elevations,
and subspecies identification would likely clear much of this up (but I’m out
of time/energy to do that within a SACC proposal). Hellmayr (1929) did not pick up on the
possibility of austral migrants messing up the distribution. Follow-up
fieldwork in the region might be highly productive. This is the region where lowland subspecies
of Thamnophilus caerulescens meet Andean subspecies, with connecting
populations with intermediate phenotypes and genotypes (Brumfield papers), and
where the same thing appears to be happening in other taxa that have similar
distributions (e.g. Pyriglena leuconota).
The sampling and analysis
of songs and calls needs to be done rigorously, with all taxa sampled. The Piranga I know, including northern
Hepatic, respond vigorously to playback, so careful playback experiments might
be illuminating. Eyal Shy’s dissertation
on North American Piranga songs needs to be read for guidance, as well
as his several subsequently published papers on geographic variation in song within
Summer (P. rubra) and Scarlet (P. olivacea) tanagers. The complexity of the structure of Piranga
songs requires quantitative analyses of their differences with a large sample
size.
I went into this issue
fairly confident that I would find sufficient anecdotal evidence that in
aggregate would make a case for elevating two or three of these subspecies groups
to species rank, but was unable to do so.
The deeper I went, the more complexity was unveiled. Although I am certain that once all the data
are available, we will have evidence for multiple species in the group, I would
be extremely reluctant to change current taxonomy without having a firm
foundation..
Let’s break down the
voting on this proposal as follows:
A YES vote means you
are in favor of splitting up Piranga flava into 2, 3, 4 (or more?)
species, with the precise breakdown to be determined in subsequent voting
round. Note that any vote on
extralimital hepatica would be strictly advisory to NACC.
A NO vote means leave
as is for now.
I recommend a NO
vote. This is a juicy project waiting
for a thorough analysis. I’m not opposed
to piecemeal taxonomy, but I really can’t find any convincing evidence for any
of the splits, as outlined in the details.
I see no immediate rush to resolve this one using fragmentary,
unsatisfactory evidence. Our current
taxonomy certainly masks species-level diversity, but any changes to it could
be considered just as misleading if not fortified by data. Tough decision.
English names: Many classifications that recognize 3 species
use Northern Hepatic-Tanager, Highland Hepatic-Tanager, and Lowland
Hepatic-Tanager for the three groups. They
have had traction in the literature since Isler and Isler (1987). But they strike me as particularly ugly, for
some reason. If this proposal passes,
then I recommend a separate proposal on English names, which would also give us
time for other choices to emerge. For
example, I would consider retaining Hepatic Tanager for the northern group,
contrary to our usual policy for parent-daughter splits, and using Tooth-billed
and Red for the other two groups, just because they have historical precedent,
albeit inconsistent (and not because they are particularly appropriate), and
this is the way IOC/xeno-canto do it.
Literature (partial)
BOESMAN, P. Notes on the
vocalizations of Northern Hepatic-tanager (Piranga hepatica), Highland
Hepatic-tanager (Piranga lutea) and Lowland Hepatic-tanager (Piranga
flava). https://birdsoftheworld.org/bow/ornith-notes/JN100386
BURNS, K. J. 1998.
Molecular phylogenetics of the genus Piranga: implications for
biogeography and the evolution of morphology and behavior. Auk 115: 621–634.
HERZOG, S. K, R. S. TERRILL.
A. E. JAHN, J. V. REMSEN, J V, O. Z. MAILLARD, V. H.GARCÍA-SOLÍZ, R. MACLEOD, A. MACCORMICK,
J. Q. VIDOZ, C. C. TOFTE, H. SLONGO, O. TINTAYA, M., AND J. FJELDSÅ,
J. 2016. Birds of Bolivia : field guide. Asociación Armonía, Santa Cruz de la Sierra,
Bolivia.
ISLER, M., AND P.
ISLER. 1987. The Tanagers, Natural History, Distribution, and Identification.
Smithsonian Institution, Washington, D.C.
MANTHEY,
J.D., L. C. CAMPILLO, K. J. BURNS, AND R. G. MOYLE. 2016.
Comparison of target-capture and restriction-site associated DNA
sequencing for phylogenomics: a test in cardinalid tanagers (Aves, genus: Piranga). Systematic Biology 65: 640–650,
ZIMMER, J. T. 1929.
A study of the Tooth-billed Red Tanager Piranga flava. Field Museum Natural History, Zoological
Series 17 (5): 169-219.
Van Remsen, June 2022
_______________________________________________________________________________
Comments
from Stiles:
“ Definitely vote NO on this one: this is a
particularly glaring example of the problems arising from insufficient taxon
sampling, with apparently conflicting results from plumage, genetics, and
vocalizations producing an undecipherable conundrum.”
Comments from Robbins: “NO. You have distilled a complex problem down to
where one can at least begin to understand the issues across multiple lines of
data. As you point out, much more data across the entire complex is needed
before we can start elevating taxa to species level. At this point, I concur with your
recommendation of a NO vote.”
Comments from Claramunt: “NO. The new data is just too
sparse to be informative regarding species limits in this widespread and
variable species.”
Comments
from Areta:
“NO. I think that Van´s recommendation is spot on: we
know the currently taxonomy is defective and masking species-level
differentiation within P. flava. But
we lack solid data to make informed decisions as to how the different species
should be circumscribed. The main problems are clear, but the data at hand
falls short to solve them.”
Comments from Pacheco: “NO. I agree with Van's recommendation. The information available for the splits in Piranga – suggested by several sources – is insufficient. Genetic and vocal analyzes have a very low N and unsatisfactory geographic coverage.”
Comments
from Bonaccorso:
“NO. The geographic, taxonomic, and vocal sampling
available is too sparse to make an informed decision.”
Comments from Zimmer: “NO,
somewhat reluctantly, because I think there’s enough smoke swirling around this
complex, that there has to be a fire someplace!
My field experience has told me for decades now that there has to be
more than one species of “Hepatic Tanager”, but, clearly, the evidence is just
too fragmented, and the sampling (be it genetic, vocal or even morphological)
is too weak, with too many gaps, so that basically, we don’t know what we don’t
know. Complex problems usually defy
simple fixes, and I think that’s the case here.
As Van states, this complex is “begging for dissertation level
research.” A couple of observations that
I would just throw out there: 1) To me, testacea is very different vocally,
particularly from nominate hepatica,
both in terms of the unique quality of its common twittering call, and, in the
relative frequency with which it delivers its various vocalizations, as
compared to other taxa in the complex, again, particularly, when compared to
nominate hepatica. I hear that latter taxon singing more than I
hear it delivering its husky, single-noted “CHUCK” calls. With testacea,
the case is a polar opposite, and I hear the unique calls far more frequently
(and when delivered, given more repetitively, in rapid-fire sequence) than I
hear them give the occasional song. To
me, it’s somewhat analogous to situations we’ve commented on before, such as
the Lophotriccus galeatus complex, or
the Zimmerius gracilipes/acer
complex, where populations on the N and S banks of the Amazon may not differ
appreciably in vocalizations, but they do differ in syntax and the relative
frequency with which different vocalizations are given. 2) With respect to the subtle plumage
distinctions between the various subspecies-groups, and Zimmer’s quoted belief
that individual variation swamped any perceived geographic structure to plumage
variation: I don’t have access to the
HBW plate that Van partially reproduced in the proposal, and that partial
reproduction doesn’t include illustrations of extralimital (for SACC) nominate hepatica, but, it has always been my
experience that NONE of the members of either the lutea-group (including haemalea)
or the flava-group with which I am
personally familiar approaches the distinctly dark-cheeked look of male &
female nominate hepatica. That character, by itself, sets hepatica apart, at least
morphologically, from all of the others, in my experience. These types of distinctions in facial
patterns are often obscured by specimen preparation, causing them to be
overlooked or downplayed by someone used to working with skins (as opposed to
seeing the birds in life) and/or by illustrators relying on skins for reference
material.”
Comments
from Jaramillo:
“YES – This proposal has already gone down, but I will go in here with a yes
vote perhaps to encourage someone out there to take this on as a project? I
think we all agree that there is almost certainly more than one species here,
and likely multiple. The uncertainty is how do divide them up because the data are
still lacking. But Yes, I do think there are multiple species, and I would be
willing to chop this up in some way…even if piecemeal and incomplete. I don’t
like to wait for the full story because that story sometimes never comes. But
we do have part of the story.”