Proposal (949) to South
American Classification Committee
Treat Caryothraustes brasiliensis as a separate species
from C. canadensis
Background: The species we treat as Caryothraustes
canadensis (Yellow-green Grosbeak) consists of four subspecies (1) simulans
of the Darién, extreme e. Panama, (2) nominate canadensis of the Guianan
Shield region and other regions locally in northern and eastern Amazonia, (3) frontalis
from e. Brazil (Paraíba, Pernambuco, Alagoas), and (4) brasiliensis of
southeastern Brazil (Bahia to RdJ). They differ primarily in head pattern,
especially with respect to forehead color, and degree of overall plumage
brightness. They have been treated as a
single species, as far as I can find, throughout their history (e.g. Hellmayr
1938, Paynter 1970, Sibley & Monroe 1990, Dickinson & Christidis 2014,
etc.).
Tangentially,
I can’t think of any other species that has such a distribution pattern, at
least when you add an isolated population from the Darién– very interesting.
New
information:
Tonetti et al. (2017) analyzed vocal,
plumage, morphometric, and genetic data of the three South American subspecies. They generated maximum-likelihood and
Bayesian trees of hypothetical relationships based on mtDNA (ND2; 29 samples;
only 1 of simulans but otherwise fairly evenly distributed). Otherwise, simulans was not included
in the quantitative analysis because of lack of access to specimens. They quantified standard song parameters
from 52 recordings, and morphometric and plumage variation (color catalog
names) using 163 study skins.
They
found that that the three South American subspecies are diagnosable units based
on coloration, and from photos, also tentatively concluded that isolated simulans
is also diagnosable; their two populations of nominate canadensis show
some possibly diagnostic color variation that is being further investigated. Morphometric analyses showed limited variation
among the three South American subspecies.
They found no differences in vocalizations: no significant differences
were found among them in any of the parameters measured. The ND2 gene trees produced 4 clades: 2
clades within nominate canadensis; simulans; and frontalis
+ brasiliensis. Here’s the
Bayesian tree (ML tree had similar topology):
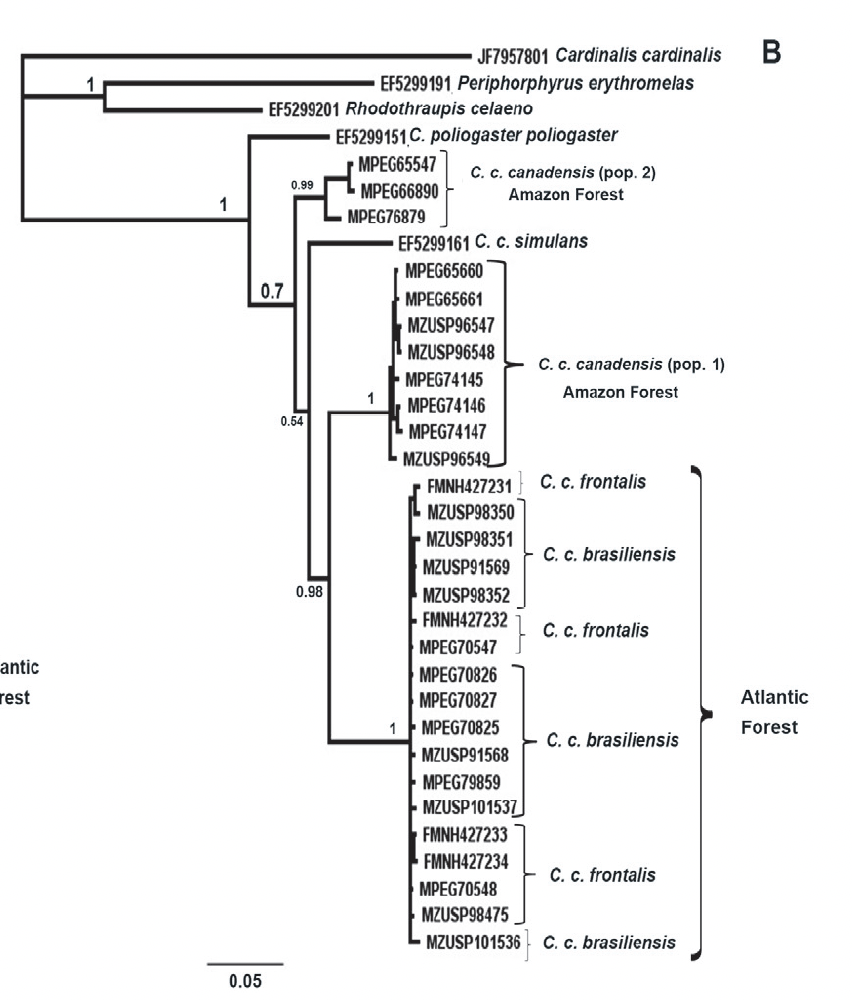
Things
to note: one group of three specimens from nominate canadensis are
sister to all samples of the other subspecies as well as the rest of the
nominate canadensis samples. From
their table, these three come from a locality in Maranhão and two in Pará, one
of which is from the Xingu. I can’t
figure out from their Fig. 4 map where all the other samples come from, and
there do not seem to be enough place markers to account for them. That distant, isolated, trans-Andean simulans
falls inside canadensis is curious.
Although frontalis and brasiliensis are diagnosable based
on extent of black on forehead color, they are interdigitated in terms of ND2.
The
authors taxonomic conclusions are that their data support recognition of two
species. Hand-waving about whether
oscine song can be used as a taxonomic character evidently is used to dismiss
the non-diagnosability in voice. The
genetic data on their own show that canadensis is paraphyletic with
respect to brasiliensis, but this is not discussed, or perhaps I am
missing where that is discussed. That
there is a 5-6% p-distance between the two populations of canadensis
and simulans vs. frontalis or brasiliensis is evidently
the primary basis for recognizing two species.
Tangent:
They recommended synonymizing frontalis with brasiliensis based
on lack of diagnosability with respect to mtDNA. However, frontalis is diagnosable
based on plumage, as their data clearly show, so the correct interpretation of
their results is that it is a valid taxon (contra those who expect subspecies
to be diagnosable on the basis of a few neutral loci). In fact, that diagnosability was so obvious
that in the first draft of this proposal I missed the conclusion of Tonetti et al. that frontalis was not a valid
taxon based on lack of reciprocal monophyly.
Rafael Lima (pers. comm.) alerted me to this misinterpretation, and he recently
published (Lima 2022) what I would consider the correct interpretation of the
data. With the reasonable assumption
that the plumage differences have a genetic basis, I take this opportunity
again to point out that recognizing these diagnosable differences taxonomically
is important and that lack of geographic structure in patterns of variation of
a few neutral loci should not mask these potentially important patterns of
phenotypic variation. See Patten and
Remsen (2017) for the full sermon.
Discussion: The paper nicely establishes
that the four described taxa are diagnosable units and thus valid
subspecies. It also reveals that there
may be an undescribed taxon within canadensis based on genetic data that
evidently corresponds to some plumage variation (to be investigated). Although the paper recommends that brasiliensis
(including frontalis) be treated as a separate species from canadensis
(including simulans) based on ND2 distance data, I am unable to follow completely
the logic for that given that the major genetic break, at least using ND2, is between
two populations of canadensis, and that using those same genetic data, C.
canadensis would be paraphyletic.
Philosophically, I am further opposed to recognizing species-level taxa
based on an arbitrary break on the continuum of genetic divergence, especially
when based on a single neutral locus.
My
interpretation of the results is that there is no evidence for treating brasiliensis
as a separate species, but rather plenty of evidence, both vocal and genetic,
for treating all taxa as a single species under the BSC, with at least four
valid subspecies (or PSC species).
Recommendation: I recommend a NO vote
on this proposal for reasons stated in previous paragraph.
Literature
cited (other than standard references):
Lima,
R. D. 2022. On the validity of Caryothraustes canadensis frontalis
(Hellmayr, 1905) (Aves: Cardinalidae).
Zootaxa 5165 (1): 144–150.
Van Remsen, June 2022
_______________________________________________________________________________
Comments
from Robbins:
“NO, for reasons outlined in proposal.”
Comments
from Claramunt:
“NO. I agree with Van, I don’t see the evidence for the split in
the mtDNA data. At least plumage and mtDNA show conflicting patterns.”
Comments
from Areta:
“NO. Some claims by Tonetti et al. (2017) are
perplexing. For example, their proposition to synonymize the diagnostic
black-fronted frontalis with brasiliensis based on their lack of
reciprocal monophyly in a mitochondrial gene (0.3 p distance), as highlighted
by Van and further shown by Lima (2022). The vocal analyses are too
coarse-grained to be informative. The phylogenetic relationships must be taken
with a grain of salt, given the low support of several clades (e.g., the
non-sister relationship of the two Amazonian, phenotypically similar
"populations"). However, the deep ND2 genetic divergences among all
forms (always above 5.5% for any pair; except for frontalis-brasiliensis)
caught my attention and beg for an explanation (as does the low divergence of frontalis-brasiliensis: might this be a case of mitochondrial capture?). A
paper by Bocalini et al. in prep is mentioned by Tonetti
et al. (2017), which would provide genomic data and presumably more detailed
phenotypic characterizations could hold the key to solve the taxonomy of Caryothraustes canadensis. Until then,
there are enough uncertainties here as to recommend caution and not adopting
any split in the group.”
Comments from Stiles: “NO for now, pending the whole-genome analysis of these that is apparently in the works.”
Comments
from Lane:
“NO. For reasons outlined by others, this suggested
split lacks important backing to make it seem necessary at this point.”
Comments from Rafael Lima: “I have just one more comment
about Caryothraustes — I saw that Areta and Stiles mentioned the "Bocalini et al.
in prep" paper that was cited by Tonetti et al.
(2017). The article in question has already been published:
Bocalini, F., Bolívar-Leguizamón, S. D., Silveira, L. F., & Bravo, G. A.
(2021). Comparative phylogeographic and demographic analyses reveal a congruent
pattern of sister relationships between bird populations of the northern and
south-central Atlantic Forest. MPE 154: 106973.
https://doi.org/10.1016/j.ympev.2020.106973
“The results of this paper concerning
Caryothraustes may be
worth commenting on the proposal, particularly those regarding
simulans (which,
possibly, may be best treated with C.
poliogaster rather than as a member of
the C. canadensis
complex).”
Comments from Bonaccorso: “NO.
For the reasons stated in the proposal. For me, it is bizarre that
they propose lump simulans into canadensis,
regardless of the tree structure (resulting from a neutral marker, but still
the only genetic data available).”
Comments
from Pacheco:
“NO. In view of the arguments put forward here,
caution is needed when adopting the split.”