Proposal (872) to South American Classification Committee
Change
species limits in Magnificent Frigatebird Fregata
magnificens
Background: The frigatebirds, with
their great plumage similarity among comparable ages and sexes, as well as the
very different immature stages over a period of several years, have long been
the subjects of taxonomic confusion. Few avian groups with so few species can
have had so many words, photos, and illustrations dedicated solely (and often
futilely) to their identification. Nevertheless, many early authors thought a
single species inhabited pan-tropical oceans, and though several taxa had by
then been named, only two species were recognized in the Catalogue of the Birds in the British Museum (Ogilvie-Grant 1898).
Two forms, a larger and smaller one, were recognized to occur on the Galapagos
by Ridgway (1897), but Rothschild and Hartert (1899) believed they intergraded
completely and therefore were not worthy even of subspecies status, until
Mathews (1914), on the basis of study of British Museum specimens, recognized
that two distinct frigatebird taxa breed in the Galapagos Islands. Among the
several new Fregata taxa he described
from around the tropics was Fregata minor
magnificens Mathews, 1914, which he considered a subspecies of Great
Frigatebird F. minor (Mathews 1914,
1915). Rothschild (1917) then reconsidered his earlier position and clarified
that magnificens should be treated as
a separate species, not a subspecies of minor.
Since then, magnificens and minor have been generally recognized as
full species that are mostly allopatric except on the Galapagos, and subsequent
authors have generally recognized five species of frigatebird (e.g., Lowe
1924). Two or three subspecies are often recognized for F. magnificens, the nominate in the Galapagos, rothschildi in most of the rest of the range, and lowei for the
highly isolated population of the Cape Verde Islands (Swarth 1933, AOU 1957,
del Hoyo and Collar 2014). In many other treatments, including most recent
ones, however, magnificens is treated
as monotypic (AOU 1931, Hellmayr and Conover 1948, Dorst and Mougin 1979,
Dickinson and Remsen 2013, Clements et al. 2019, Gill et al. 2020). The AOU
(e.g., AOU 1889, 1895, 1910) long recognized just a single regional species,
now known as the Magnificent Frigatebird F.
magnificens, but three species are now included in the expanded NACC region
(AOU 1983, Chesser et al. 2019), and four in the SACC region (Remsen et al.
2020).
New information: Hailer et al. (2011)
found that over most of their New World range, F. magnificens has high levels of gene flow, including across the
Isthmus of Panama, which usually serves as a barrier to other taxa of seabirds.
However, despite predictions based on the extraordinary vagility of
frigatebirds, the Galapagos population was found to be strongly genetically
differentiated from the other populations (see screenshots below of Figs. 2 and
3 from Hailer et al. 2011). The Galapagos population must therefore have been
genetically isolated for at least a few hundred thousand years (Hailer et al.
2011).
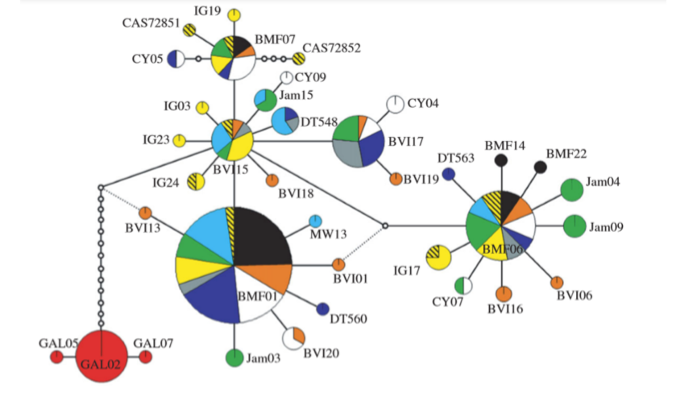
Fig.
2 from Hailer et al. (2011). Parsimony network of mtDNA sequences, Galapagos in
red.
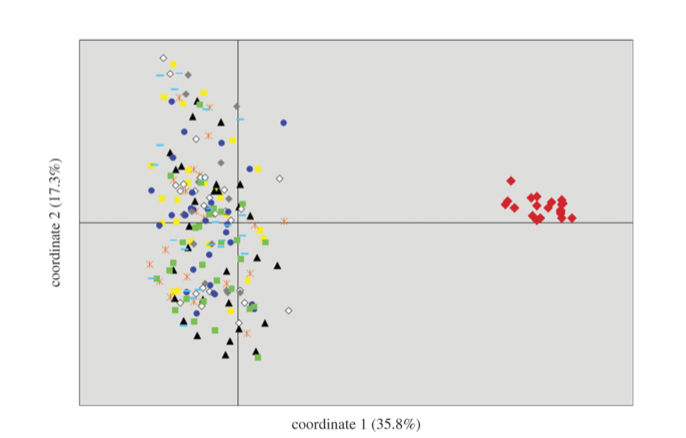
Fig.
3 from Hailer et al. (2011). PCA of microsatellite genotypes, Galapagos in red.
Further,
in a study of population structure primarily of Atlantic and Caribbean
populations, Nuss et al. (2016) found that, although
Brazilian and Caribbean populations were genetically isolated from one another,
the geographically interposed population from French Guiana (Grand Connétable) shared haplotypes with both regions. As shown
by Hailer et al. (2011), the Galapagos population had highly divergent
haplotypes (see screenshot of Fig. 2 from Nuss et al.
2016, below). In a study of Mexican F.
magnificens populations, Rocha-Olivares and González-Jaramillo (2014) found
lower levels of gene flow between colonies and especially between ocean basins
than did Hailer et al. (2011), and offered explanations for this discrepancy,
including more individuals sampled, more peripheral sampling locations, and
greater geographical distances in their more recent study.
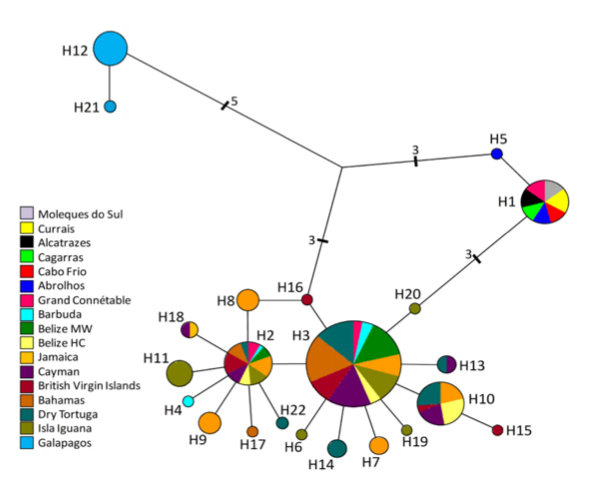
Fig.
2 from Nuss et al. (2016). Median-joining network,
mtDNA haplotypes.
Despite
the genetic divergence of the Galapagos F.
magnificens population, the same
haemoprotean parasite, Haemoproteus iwo,
occurs in F. minor as well as F. m. magnificens from Galapagos and
multiple localities within the range of F.
m. rothschildi (Clade B of Fig. 1 in Levin et al. 2011, see screenshot
below). This non-congruence in divergence levels between parasite and host
suggests that transfer by hippoboscid flies (e.g. Olfersia) may take place between non-breeding frigatebirds at
communal roosts, as well as at colonies. There is some movement of non-breeding
Galapagos Magnificent Frigatebirds to Central America, where dead or emaciated
birds banded in the Galapagos have been recovered (according to a pers. comm.
in Hailer et al. 2011, details not provided). The haemoprotean parasite’s lack
of divergence may be considered confirmatory of the mixing of frigatebird
populations outside of the breeding colonies.
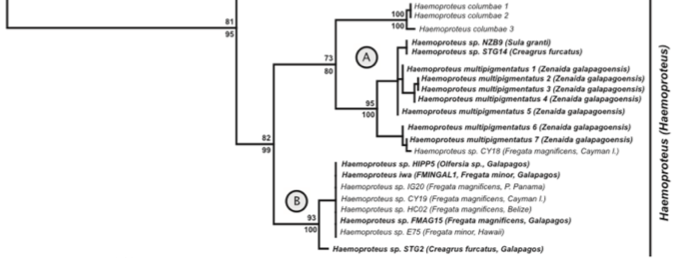
Fig.
1 of Levin et al. (2011). Relevant part of ML tree of haemosporidian cytb.
Several
external measurements taken of Magnificent Frigatebirds (5 of each sex from the
Galapagos, 11 and 16 from non-Galapagos specimens) corroborated that, in wing
and inner and outer tail dimensions, and culmen of females, the Galapagos
population is significantly larger (Hailer et al. 2011). My informal inspection
of many eBird photos (selecting only those in non-foreshortened view) seems to
confirm that the tail is relatively longer in Galapagos birds. Otherwise, I
cannot see any plumage or soft part color differences and am unaware of any
that have been reliably suggested.
The
authors of the above studies are in general agreement that sex-biased dispersal
(with males exhibiting site fidelity) and female mate choice for the complex
male mating rituals of frigatebirds is most likely to explain the patterns
seen, especially the genetic distinctness of the Galapagos population. However,
none presented or referenced any data on differences in e.g. display or
vocalizations. I located only one online recording from the Galapagos, which
did not prove useful, though there must be recordings in compilations and
private collections. It seems apparent that isolating mechanisms beyond simple
geographical isolation must be operating, otherwise the Galapagos population
would be experiencing gene flow with mainland birds.
The
Cape Verde population named F. m. lowei has not been included in the above studies, but
as of 2012 only one bird of each sex appeared to be present at the former
colony (Suarez et al. 2012), due to persecution. These authors indicated their
intention to conduct genetic and mensural analysis of the distinctiveness of
this virtually extinct and highly isolated population.
Note
that Olson (2017) has recently espoused treatment of Lesser Frigatebird Fregata ariel as two species, as the
form trinitatis now restricted to
Trindade Island and known from St. Helena by fossils has different proportions
(stouter wing and bill) and putative plumage differences in immature stages.
There are parallels with the case of Magnificent Frigatebird, but genetic data
that would bolster the case for specific status of trinitatis appear to be lacking. A proposal (SACC#768, https://www.museum.lsu.edu/~Remsen/SACCprop768.htm) to split trinitatis was rejected in favor of
further evidence.
Effect on AOS-NACC and
SACC areas: If
split, the Galapagos population would retain the specific epithet magnificens, and thus the widespread
frigatebird of the Americas would become Fregata
rothschildi. There would also obviously be English name issues. In addition,
there are evidently records of Galapagos birds from somewhere in Central
America, so details would have to be located for the NACC area, and a new
species added to both regional checklists.
It
should be added that, even if this proposal does not pass, this species clearly
should not be considered monotypic by global checklist authorities.
Recommendations:
Although
it would be ideal if there were behavioral studies and analyses of the
courtship display vocalization repertoire that established the existence of
premating isolating mechanisms between Galapagos and mainland birds, these are
not available to my knowledge. What we do know is that there is little if any
gene flow, despite movement of at least some non-breeding individuals from the
Galapagos to the mainland, which I take to be prima facie evidence that
speciation has occurred. Please vote separately for each option.
A.
A YES vote for (A) would be to split F.
m. magnificens, with the resultant daughter species F. magnificens of the Galapagos and F. rothschildi in the remainder of the range (whether or not lowei of Cape
Verde is recognized).
If
(A) passes, English name issues arise. Obviously, Magnificent Frigatebird could
be retained for the vastly more widespread rothschildi
but it would no doubt cause confusion, given the retention of magnificens by the Galapagos form.
(Consider though that we have all learned to live with Great Frigatebird being Fregata minor, while Lesser is F. ariel.) Another option might be
American Frigatebird, but of course Galapagos are part of the Americas, and in
fact American Man-O-War Bird was used by Hellmayr and Conover (1948) for their
monotypic, widespread F. magnificens
(including the Galapagos population). I recommend retention of Magnificent
though as being the least disruptive option.
B.
A YES vote for (B) would be to retain Magnificent Frigatebird for Fregata rothschildi.
Galapagos
Frigatebird would be my suggestion for F.
magnificens s.s.
C. A YES vote for (C) would be for Galapagos
Frigatebird F. magnificens.
Literature Cited:
American
Ornithologists’ Union (1889). Check-list of North American Birds. First Edition,
Abridged. American Ornithologists’ Union.
American
Ornithologists’ Union (1895). Check-list of North American Birds. Second
Edition, Revised. American Ornithologists’ Union.
American
Ornithologists’ Union (1910). Check-list of North American Birds. Third
Edition. American Ornithologists’ Union.
American
Ornithologists’ Union (1931). Check-list of North American Birds. Fourth
Edition. American Ornithologists’ Union.
American
Ornithologists’ Union (1957). Check-list of North American Birds. Fifth
Edition. American Ornithologists’ Union.
American
Ornithologists’ Union (1983). Check-list of North American Birds. Sixth
Edition. American Ornithologists’ Union.
Chesser, R. T., K. J. Burns, C. Cicero, J. L. Dunn, A. W. Kratter, I. J.
Lovette, P. C. Rasmussen, J. V. Remsen, Jr., D. F. Stotz, and K. Winker (2019).
Check-list of North American Birds (online). American Ornithological Society. http://checklist.americanornithology.org/taxa
Clements, J. F., T. S. Schulenberg, M. J. Iliff,
S. M. Billerman, T. A. Fredericks, B. L. Sullivan, and C. L. Wood (2019). The
eBird/Clements Checklist of Birds of the World: v2019. Downloaded from https://www.birds.cornell.edu/clementschecklist/download/
del Hoyo, J., and N. J.
Collar (2016). HBW and BirdLife International Illustrated Checklist of the
Birds of the World. Volume 2: Passerines. Lynx Edicions, Barcelona.
Dorst, J., and J.-L.
Mougin (1979). Order Pelecaniformes.
Pp. 155–193 in: Mayr, E. and G. W. Cottrell (Editors). Check-list of the Birds
of the World. Volume 1, Ed. 2. Harvard University Press, Cambridge, MA.
Dickinson, E. C., and
J. V. Remsen, Jr. (Editors) (2013). The Howard and Moore Complete Checklist of
the Birds of the World. 4th edition. Volume One. Non-passerines.
Aves Press Ltd., Eastbourne, UK.
Gill, F., D. Donsker,
and P. C. Rasmussen (Editors) (2020). IOC World Bird List (v 10.2). DOI
10.14344/IOC.ML.10.2. http://www.worldbirdnames.org/
Hailer, F., E. A.
Schreiber, J. M. Miller, I. I. Levin, P. G. Parker, R. T. Chesser, and R. C.
Fleischer (2011). Long-term isolation of a highly mobile seabird on the
Galapagos. Proceedings of the Royal Society B 278:817–825.
Hellmayr, C. E., and B.
Conover (1948). Catalogue of Birds of the Americas and Adjacent Islands. Vol.
13, Part I, No. 2. Publication 615, Field Museum of Natural History, Chicago.
Levin, I. I., G.
Valkiunas, D. Santiago-Alarcon, L. L. Cruz, Tatjana A. Iezhova, S. L. O’Brien,
F. Hailer, D. Dearborn, E. A. Schreiber, R. C. Fleischer, R. E. Ricklefs, and
P. G. Parker (2011). Hippoboscid-transmitted Haemoproteus parasites (Haemosporida) infect Galapagos pelecaniform
birds: evidence from molecular and morphological studies, with a description of
Haemoproteus iwa. International
Journal for Parasitology 41:1019–1027.
Lopez-Suarez, P., C. Hazevoet, and L. Palma (2012). Has the Magnificent
Frigatebird Fregata magnificens in
the Cape Verde Islands reached the end of the road? Zoologia Caboverdiana
3:82–86.
Lowe, P. R. (1924).
Some notes on the Fregatidae. Novitates Zoologicae 31:299–313.
Mathews, G. M. (1914).
On the species and subspecies of the genus Fregata.
The Austral Avian Record 2:117–121.
Mathews, G. M. (1915).
The Birds of Australia. Volume 4. Witherby, London.
Nuss, A., C. J. Carlos, I.
B. Moreno, and N. J. R. Fagundes (2016). Population genetic structure of the
Magnificent Frigatebird Fregata
magnificens (Aves, Suliformes) breeding colonies in the Western Atlantic
Ocean. PLoS ONE 11:e0149834.
Ogilvie-Grant, W. R. (1898). Catalogue of the Birds in the British
Museum. Vol. 26. Trustees, London.
Olson, S. L. (2017) Species rank for the critically endangered Atlantic
Lesser Frigatebird (Fregata trinitatis).
Wilson Journal of Ornithology 129:661–675.
Remsen, J. V., Jr., J. I. Areta, C. D. Cadena, S. Claramunt, A.
Jaramillo, J. F. Pacheco, J. Perez Emán, M. B. Robbins, F. G. Stiles D. F.
Stotz, and K. J. Zimmer (Version 11 February 2020). A Classification of the
Bird Species of South America. American Ornithological Society.
http://www.museum.lsu.edu/~Remsen/SACCBaseline.htm
Ridgway, R. (1897). Birds of the Galapagos Archipelago. No. 1116.
Proceedings of the United States National Museum 19:459–670.
Rocha-Olivares, A. and
M. González-Jaramillo (2014). Population genetic structure of Mexican
Magnificent Frigatebirds: an integrative analysis of the influence of
reproductive behavior and sex-biased dispersal. Revista Mexicana de
Biodiversidad 85:532–545.
Rothschild, W. (1917). On the genus Fregata.
Novitates Zoologicae 22:145–146.
Swarth, H. S. (1933).
Frigate-birds of the west American coast. Condor 35: 148–150.
Pamela
C. Rasmussen, August 2020
Comments
from Areta:
“NO. The fact that there are some minor morphological measurements and an
unimpressive 0.88% of genetic divergence seems not enough to split magnificens from rothschildi. It certainly is
interesting that there is no apparent gene flow between Panamanian Pacific
populations and the Galapagos, which one might well prima facie expect based on
the high vagility of frigatebirds. But
the lack of gene flow does not necessarily equates with the existence of a
separate species. The connectivity seems
much better in the Caribbean than on the Pacific, so the patterns of flow on
the Caribbean are not that surprising. I would like to see what happens when
populations breeding further south along the Pacific (e.g., in Ecuador) are
sampled. Will they show evidence of gene flow? Will they reduce the level of
genetic differentiation thought to exist between continental and Galapagos
populations?”
Comments
from Stiles:
“NO, for now. The complete distinction in haplotypes clearly indicates no
interbreeding between the Galápagos and all mainland populations studied so
far. Given that magnificens s. l.
breeds on islands along the Pacific coast at least from Costa Rica south to
Peru, genetic data from these populations would seem to be the missing piece in
the puzzle. Although I think that Pam is probably right, I would prefer to
await data from these populations. (At least in Costa Rica, occasional
individuals of Fregata do cross over
the country between the Caribbean and the Pacific).”
Comments
from Claramunt:
“NO. Very suggestive of species status but I agree
with Nacho that populations along the coast of Ecuador should be evaluated
before making such a traumatic change.
Comments
from Robbins:
“NO. I agree that Pacific populations in
Central and northern South America should be sampled to provide a better
picture before making a change.”
Comments from Zimmer: “NO
for now. As others have already
expressed, I think we need more sampling from more sites along the Pacific
Coast of the Americas to sort out the nature of gene flow and ascertain what
the bottlenecks are. Meanwhile, some
concrete evidence of potential premating isolating mechanisms (such as in
display/courtship vocalizations) between Galapagos birds and other populations
of magnificens, would be particularly
helpful.”
Comments from Remsen: “NO. Divergence in mtDNA is insufficient evidence
for species rank. Nor does lack of gene
flow between allopatric BREEDING populations, in my opinion, even in something
as vagile as Fregata; parapatric or sympatric, yes, then lack of gene
flow is prima facie evidence of speciation.
Nor does slightly larger body size.
Even taken together, the evidence is insufficient. Further, as pointed out by several above, the
geographic sampling scheme is inadequate.
The “bar” for species limits is pretty low in Fregata, but I
don’t see any evidence that the bar has been reached in this case.”
Comments
from Pacheco:
“NO. The suggestion of Nacho seems reasonable to me
that “key” populations along the coast of Ecuador should be assessed before
making a major change.”
Comments from Lane: “NO. As others have pointed out,
I think there are too many unsampled populations to put this case to rest.”
Comments from Jaramillo: “NO – An analysis of display and voice
needs to be done, given that flying around with a big red inflatable pouch
while making vibrating sounds is a pretty major and obvious display that these
birds use to secure a mate. I think that there should be some differences in
display once analyzed if the Galapagos birds are a different species. Note that
they are sympatric with Great Frigatebirds on some of the islands, so there
will be a “standard” for difference in displays in two clear-cut species
breeding side by side.
“Another thing to consider is that Magnificents are a species that is a nearshore marine bird.
They are not truly pelagic as Great Frigates and other species in the genus. So
it is possible that while in our heads we may think of frigatebirds as being
highly mobile, these birds may be resident and may never move farther away than
100 km from the islands. So this alone could create the lack of gene flow. Most
other populations are somewhat near adjacent populations, and island groups are
not nearly as isolated as the Galapagos.”
Comments from Bonaccorso: “NO. Although I think that the
mitochondrial DNA and microsatellite data are very convincing. That level of
genetic divergence in a population of birds from Galapagos and, especially, in
vagile organisms as Fregata, seems like very good evidence of
reproductive isolation. However, I think this proposal should include a
systematic comparison of measurements and, more importantly, courtship
vocalizations between the Galapagos population and other populations.”