Proposal (1006) to South
American Classification Committee
Treat Amazona guatemalae as a separate species
from A. farinosa (Mealy Parrot)
Note from Remsen: This
is a NACC proposal (2023-A-16) that was rejected that I am posting here, with
permissions) because it has the potential to affect the distribution and
component taxa of what we currently classify as Amazona farinosa. You can see official comments from NACC
voters here; the
vote was 7 to 5 to reject.
Background:
The Mealy Parrot (Amazona farinosa) occurs in southern
Mexico, through all of Central America, parts of northern South America and the
Amazon Basin, and also has a disjunct population in the Atlantic Forest of
Brazil. Most authorities currently recognize 3-5 subspecies of A. farinosa, which are often split into
two groups (sensu Clements et al. 2021): the Northern Mealy Parrot (A. f. guatemalae from the Caribbean
slope of southeastern Mexico to northwestern Honduras and A. f. virenticeps from the Sula Valley of Honduras to extreme w
Panama) and the Southern Mealy Parrot (A.
f. farinosa, which occurs east and south from Panama to Colombia, Peru,
Bolivia, the Guianas, and disjunctly in the Atlantic Forest of southeastern
Brazil). Although most authorities consider the Southern Mealy Parrot to be
monotypic, it is sometimes split into three subspecies, including A. f. Inornata (Panama and Colombia), A. f. chapmani (SE Peru to NW Bolivia),
and A. f. farinosa in the central
Amazon Basin and Atlantic Forest.
Until recent HBW-Birdlife and
IOC splits, the two putative species have been treated as conspecific. HBW-BL
split A. farinosa into two species
based on the following rationale.
Until
recently, [guatemalae] was considered
conspecific with A. farinosa, but
differs in its yellow vs red lower carpal edge (2); blue-suffused (or blue)
crown with broader, more heavily scaled nape feathers forming frequently or
usually ruffled ruff or cape (3); blackish vs pale bill (2); black bristles on
nares more extensive, and black shaft streaks on face (lores to below eye)
(ns1); less powdery plumage (ns1); more oblong, less circular and slightly less
broad white eye-patch (mensural score: allow 1). This split is supported by
molecular analysis (Wenner, Russello & Wright 2012).
The IOC note on this issue is:
"Northern Mealy Amazon is split from [Southern] Mealy Amazon (Wenner et
al. 2012; HBW Alive)."
As suggested above, this
proposed split is largely based on slight differences in plumage coloration
between the Northern Mealy Parrot and Southern Mealy Parrot groups with support
from population genetic data. The NACC and SACC have not yet considered these
data in voting on species limits within the Mealy Parrot complex.
New
Information:
Morphology:
This is not new information
per se, but rather a synopsis of phenotypic differences between the Northern
Mealy Parrot and Southern Mealy Parrot groups.
Ridgway (1916) determined that
phenotypic variation between Central and South American lineages was clinal
(Table 1). Although we assume that Ridgway was referring to the morphometric
measurements that appear directly above his statement about intergradation,
it’s not absolutely clear whether he was referring to morphometrics, color, or
some other aspect of phenotype.
Table 1: From Ridgway (1916),
morphometric measurements of A. f. inornata and statement beneath
stating that intergradation occurs between A. f. farinosa and A. f.
virenticeps. We are unclear based on the placement of this statement what
characters Ridgway (1916) was referring to, but believe the statement was in
reference to morphometric characters.
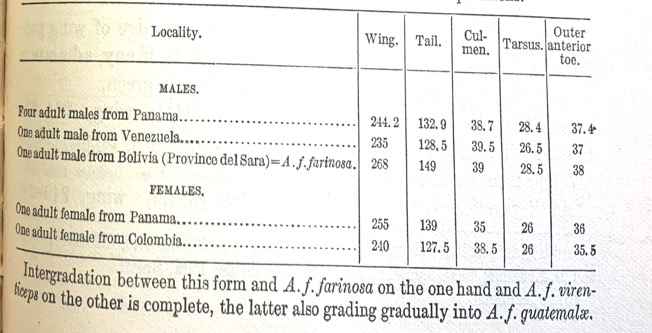
In contrast, Wetmore (1968)
noted that farinosa (inornata from Panama) averaged larger in wing, tail, culmen
(from cere), and tarsus length (Table 2). As mentioned above, the HBW split was
based on the minor plumage differences summarized here. Bill color differs
between the two, being pale in southern and blackish in northern. Crown color
is blue or suffused blue in Northern Mealy Parrot while Southern Mealy Parrot
lacks blue in the crown. Although Southern Mealy Parrots tend to show yellow in
their crowns more often, some Northern Mealy Parrots also have yellow in their crowns.
Northern Mealy Parrots also typically have more heavy scaling on their nape.
Other differences include a yellow lower carpal edge in Northern Mealy Parrot,
whereas this is red, yellow, or a combination of both in Southern Mealy Parrot.
Also, Northern Mealy Parrots tend to have more extensive bristles on nares and
shaft streaks on the face, less “powdery” plumage, and a more oblong and
narrower white eye patch compared to Southern Mealy Parrots.
Table 2. Wetmore (1968)
measurements
|
|
wing |
tail |
Culmen
from cere |
tarsus |
|
virenticeps male
(n=9) |
229.5
mm |
122.9
mm |
34.6
mm |
28.1
mm |
|
virenticeps female
(n=8) |
225.4
mm |
123.7
mm |
34.6
mm |
28.1
mm |
|
inornata male
(n=10) |
235
mm |
131.7
mm |
36.3
mm |
29.3
mm |
|
inornata female
(n=8) |
233.8
mm |
132.7
mm |
36.4
mm |
28.6
mm |
Below are Macaulay Library
photos showing variation in some of these features, especially bill, crown, and
eye ring.
Northern
https://macaulaylibrary.org/asset/433053151
https://macaulaylibrary.org/asset/439969671
https://macaulaylibrary.org/asset/465517171
https://macaulaylibrary.org/asset/432513681
https://macaulaylibrary.org/asset/417454551
Southern
https://macaulaylibrary.org/asset/364752721
https://macaulaylibrary.org/asset/422619441
https://macaulaylibrary.org/asset/364752631
https://macaulaylibrary.org/asset/406771601
Population
genetics:
Wenner et al. (2012) sequenced
two mtDNA gene regions (1,157 bp of Cyt b + COI combined) and two nuDNA introns
(1,145 bp of TGFB2 + TROP combined) to examine phylogenetic structure among the
five recognized subspecies of A. farinosa
(Fig. 1). Hellmich et al. (2021) expanded on this study to include samples
of the geographically disjunct Atlantic Forest population of the nominate A. f. farinosa. Aside from the addition
of the Atlantic Forest population, the two data sets are identical. Although
both nuDNA and mtDNA were included in these studies, the sampling matrix is
incomplete such that multiple individuals are missing data from one or more
loci or gene regions. Additionally, the number of parsimony-informative sites
in the mtDNA data set (n = 96) was far more than the nuDNA data set (n = 5),
such that the concatenated / combined phylogenetic data sets are largely driven
by information contained in the mtDNA genome.
Wenner et al. (2012) recovered
16 cyt-b haplotypes with 28 sequence differences between Northern Mealy Parrot
and Southern Mealy Parrot clades (Fig. 2). This corresponded to mtDNA distances
of 3.5–5.4% between the two clades, which translates to an approximate
divergence time of 1.75–2.7 mya during the late Pliocene to early Pleistocene.
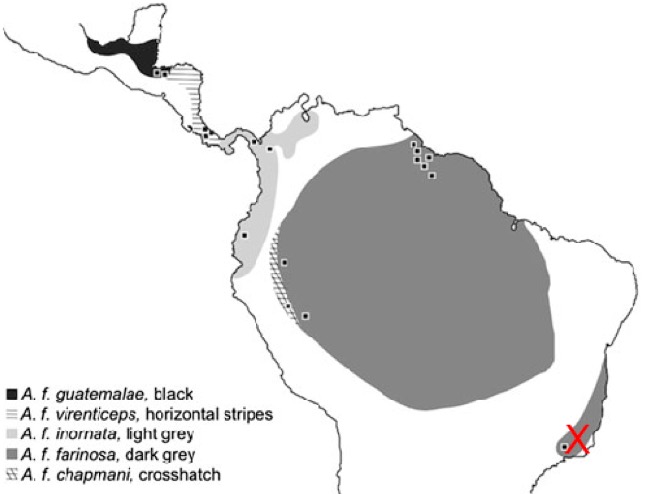
Figure
1: Sampling localities and ranges of currently recognized subspecies. Red “X”
indicates approximate locality of additional samples from the Atlantic Forest
of Brazil that were included by Hellmich et al. (2021).
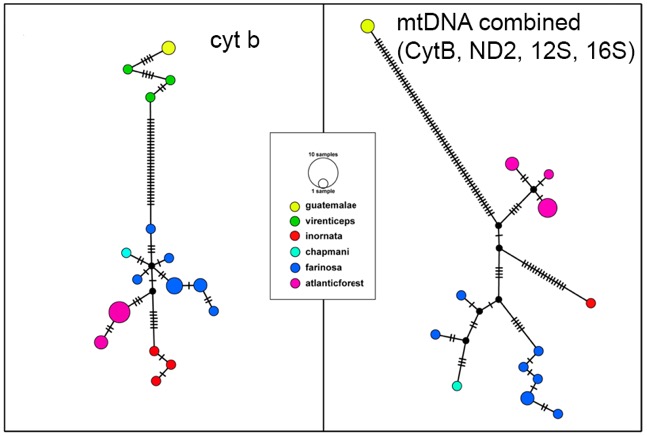
Figure 2: (Left panel) Median
joining haplotype network based on CytB data. Size of each circle corresponds
to the number of individuals sharing that haplotype and color to each clade.
(Right panel) Median joining haplotype network based on 4 mitochondrial genes
(CytB, ND2, 12S, 16S). Size of each circle corresponds to the number of
individuals sharing that haplotype and color to each clade. Ticks on each
branch represent the number of sequence differences between each haplotype.
Using their combined data set
of mtDNA + nuDNA, Wenner et al. (2012) recovered reciprocal monophyly and a
deep phylogenetic split between the Northern Mealy Parrot (virenticeps and guatemalae) and the Southern Mealy Parrot (farinosa, inornata, and chapmani).
The nuDNA tree with the highest maximum likelihood score had very low bootstrap
support for all of the nodes within the Mealy Parrot complex, essentially
producing a polytomy (Fig. 3). Additional sampling of the Atlantic Forest
population by Hellmich et al. (2021) recovered the same topology, and found
that the Atlantic Forest population formed a monophyletic group (Fig. 4).
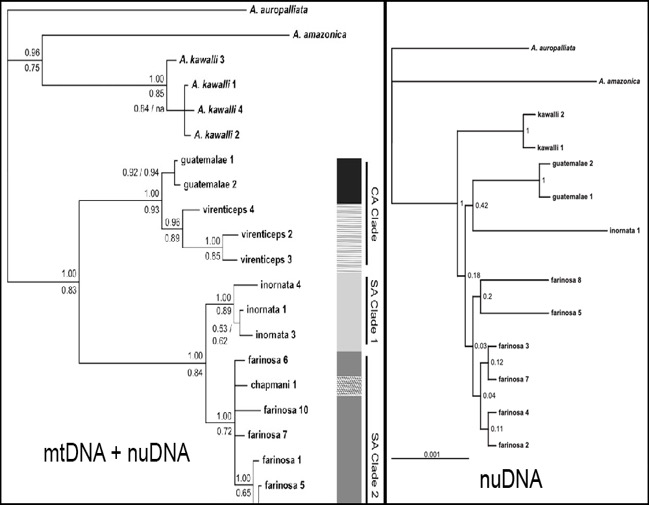
Figure 3: Phylogenies from combined mtDNA + nuDNA (left) and nuDNA alone
(right) of Mealy Parrots from Wenner et al. (2012). Posterior probabilities are
shown above each node while maximum likelihood bootstrap values are shown
below, or to the side for the nuDNA alone phylogeny.
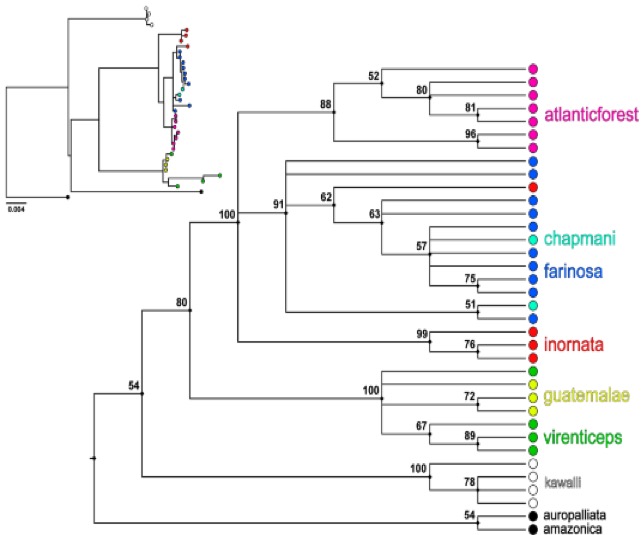
Figure 4: Maximum likelihood
majority rule consensus tree (cladogram without branch lengths) based on
combined nuDNA and mtDNA from Hellmich et al. (2021) with expanded Atlantic
Forest sampling. Numbers to the left of each node are bootstrap consensus values.
Top-left inset is a phylogram with branch lengths included that are
proportional to sequence divergence.
Vocalizations:
Hellmich et al. (2021) used
150 samples of contact calls (Fig. 5) to investigate differences in call
structure across the 5 subspecies and the Brazilian Atlantic forest
populations. They found that variation within each subspecies was as great as
between subspecies with substantial overlap in acoustic principal component
space among clades (Fig. 6). They also found no correlation between genetic
differentiation and vocal differentiation among clades.
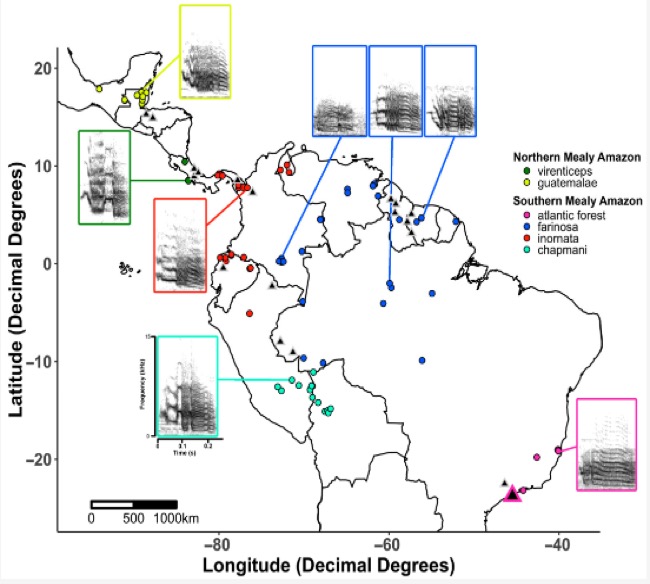
Figure 5: Map of vocal and
genetic sampling locations from Hellmich et al. (2021). Spectrograms of
representative calls from each clade are shown at their corresponding recording
location. Genetic samples from the Wenner et al. (2012) study are indicated by
grey-outlined triangles on the map. The location of the new genetic samples
included in Hellmich et al. (2021) is indicated by the purple-outlined
triangle.
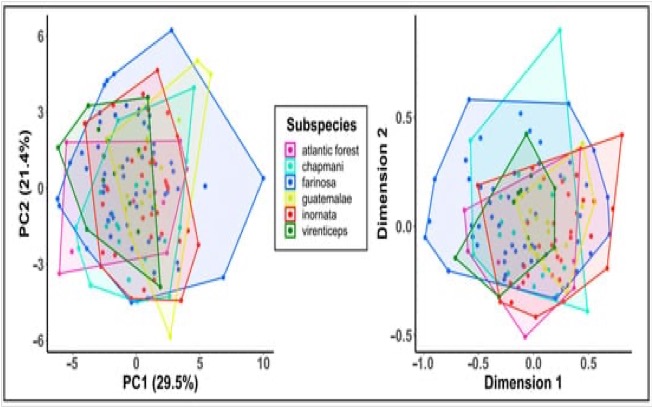
Figure
6: Acoustic variation in call data. Plots of acoustic variation in contact
calls based on principal components analysis of 27 call measures (left) and a multidimensional scaling of
spectrogram cross-correlation values (right).
The points represent individual calls, and the polygons represent the total
area occupied by each clade’s set of calls in acoustic space. This is Figure 4
from Hellmich et al. (2021).
Recommendation:
Phenotypic differences between
these groups are very slight—being limited to a few plumage characters that are
not diagnostic—and may be clinal through the Isthmus of Panama. Vocalizations
are variable throughout the complex and do not differ consistently between
northern and southern groups. Although there is substantial mtDNA divergence
(3.5–5.4%), no shared haplotypes, and reciprocal monophyly between the northern
and southern groups, there is still a lot of uncertainty regarding contact zone
dynamics. Most authorities state that the northern and southern groups are
allopatric, but the evidence for this is unclear, and the distance between them
is also unknown. Based on eBird records, the two groups appear to be separated
by a narrow gap (~50 km) in central Panama, but current sampling for genetic
analyses from the putative contact zone is sparse, and these subspecies can be
difficult to distinguish in the field. Thus, the contact zone remains largely
uncharacterized, both in terms of phenotypic and genetic differentiation. Mealy
Parrots have also been commonly held in captivity throughout the region, both
currently and historically by indigenous communities, which has increased
opportunities for escapees to come into contact.
Taken together, we feel that
although there is considerable evidence for cryptic speciation based on mtDNA
divergence, the small amount of nuDNA is largely uninformative and does not
recover the same pattern of deep reciprocal monophyly between Northern and
Southern Mealy Parrots. Furthermore, the phenotypic differences are slight
compared to other Amazona sister
species, and the potential for hybridization in the contact zone remains
unstudied. Acting conservatively, we therefore feel that Northern and Southern
Mealy Parrots should not be split.
We recommend a NO vote on this
proposal.
Literature
Cited:
Clements,
J., T. Schulenberg, S. Billerman, T. Fredericks, J. Gerbracht, D. Lepage, B.
Sullivan, and C. Wood (2021). The eBird/Clements checklist of Birds of the
World: v2021. Cornell Lab of Ornithology.
Hellmich,
D. L., A. B. S. Saidenberg, and T. F. Wright (2021). Genetic, but not
behavioral, evidence supports the distinctiveness of the Mealy Amazon Parrot in
the Brazilian Atlantic Forest. Diversity 13:273.
Ridgway,
R (1916). The birds of North and Middle America. Part 7. Bulletin U.S National
Museum 50.
Wenner,
T. J., M. A. Russello, and T. F. Wright (2012). Cryptic species in a
Neotropical parrot: genetic variation within the Amazona farinosa species
complex and its conservation implications. Conservation Genetics 13:1427–1432.
Wetmore, A. (1968). The Birds of the Republic of Panama. Part 2.
Columbidae (Pigeons) to Picidae (Woodpeckers). Smithsonian Miscellaneous
Collections 150. Smithsonian Institution, Washington, D.C.
David Vander Pluym and Nicholas A. Mason,
June 2024
_______________________________________________________________________________________________________________________________________________
Vote tracking chart:
https://www.museum.lsu.edu/~Remsen/SACCPropChart968-1043.htm
Comments
from Remsen:
“NO, as I voted in the NACC proposal, and for all the reasons given in the
proposal. There’s a potential contact
zone in Panama, and that needs to be adequately characterized.”
Comments
from Areta:
“NO: the Panama area should be rigorously screened, phenotypic
distinctions are minor, the discordance between mt and nuc DNA suggests
caution, and the vocalizations (that tend to be well-marked in Amazona species) appear as a single
cluster in the quantitative vocal analyses.”
Comments
from Robbins:
“NO, for all the reasons detailed in the proposal.”
Comments
from Jaramillo:
“YES – I will be the outlier. Back when I used to see elements of this group on
tours, I was always surprised that the Central American birds just seemed quite
different to me from the South American birds. That is not all that useful, but
obviously part of why I am more permissive with this topic. The DNA data tells
a partial story, but there is a signal there. I realize that most will likely
vote this down, but I am persuaded. I do wish that sometimes we had some
information from the parrot breeder people, I bet they see similarities and
differences between populations that we do not, and with the right context,
these could be useful in making some of these decisions.”
Comments
from Stiles:
“NO. As noted by Van and others, the central Panama area would be a contact
zone critical for separating the northern and South American groups: here,
genetic and phenotypic information are needed: are they parapatric, or do they
intergrade?”
Comments
from Lane:
“NO to splitting the two groups of A. farinosa. The evidence provided in
the proposal suggests that they are not sufficiently distinctive with respect
to one another to warrant a split. Further, I am not sure what to do with Amazona
voices. In some situations, particularly when in syntopy, they are clearly very
important characters for species recognition. But when dealing with populations
at several points within a species’ or taxon group’s range, I have been seeing
some serious issues that (what I assume is) dialect formation can introduce.
Obviously, parrots can and do learn their vocabulary, but we largely accept
that there are common vocalizations (flight calls, particularly) that are
fairly uniform over the majority of the distribution of any given species
(using the term loosely here). But, for example, I first learned the voice of Amazona
autumnalis from birds in Tamaulipas, Mexico, which sound like this (https://xeno-canto.org/28759; a very distinctive
“CHEE-colek”), and then encountered them in Belize, where they sounded
different (https://xeno-canto.org/28345; a shorter “TOE-tick”
that still has a distinctly bisyllabic structure), but I thought I still
detected enough similarity that it didn’t really faze me. Then, a few years
ago, I encountered the species on the Gulf slope of Oaxaca (intermediate
between the previous two sites), and was blown away by how different they
sounded there (https://macaulaylibrary.org/asset/79933521, which lacked any
bisyllabic construction at all)! In fact, I was misidentifying those birds as A.
farinosa guatemalae until I was able to see the source! I should note that
all three populations are considered to fall under the same, nominate,
subspecies of A. autumnalis. I realize that this proposal is about A.
farinosa and not A. autumnalis, but since these are congeners, I
think it may be useful to infer from that species that the fact that the voices
within the populations of the A. farinosa complex don’t match genetic
populations may not be at all surprising here.”
Comments
from Claramunt:
“NO. Only mitochondrial DNA suggests the split; other types of data are
ambiguous or not diagnostic.”
Comments
from Zimmer:
“NO. The conflicting
interpretations resulting from mitochondrial DNA analysis versus nuclear DNA
analysis do give pause, the morphological differences don’t (in my opinion)
meet the ‘yardstick’ test within the genus, there are no apparent diagnostic
vocal differences between the two groups, and we lack data on all of the above
from the potential contact zone in central Panama.”