Proposal (1019) to South
American Classification Committee
Treat Granatellus
paraensis as a separate species from G. pelzelni
Note: This is a
high-priority issue for WGAC.
Background: Our new SACC note on this is as follows:
34a. Del Hoyo & Collar (2016) treated the subspecies paraensis
of se. Amazonia as a separate species (Rose-bellied Chat), based in part on
vocal differences from nominate pelzelni described by Boesman (2016n).
Here
is the basic set-up. Granatellus pelzelni is considered to consist of
two subspecies:
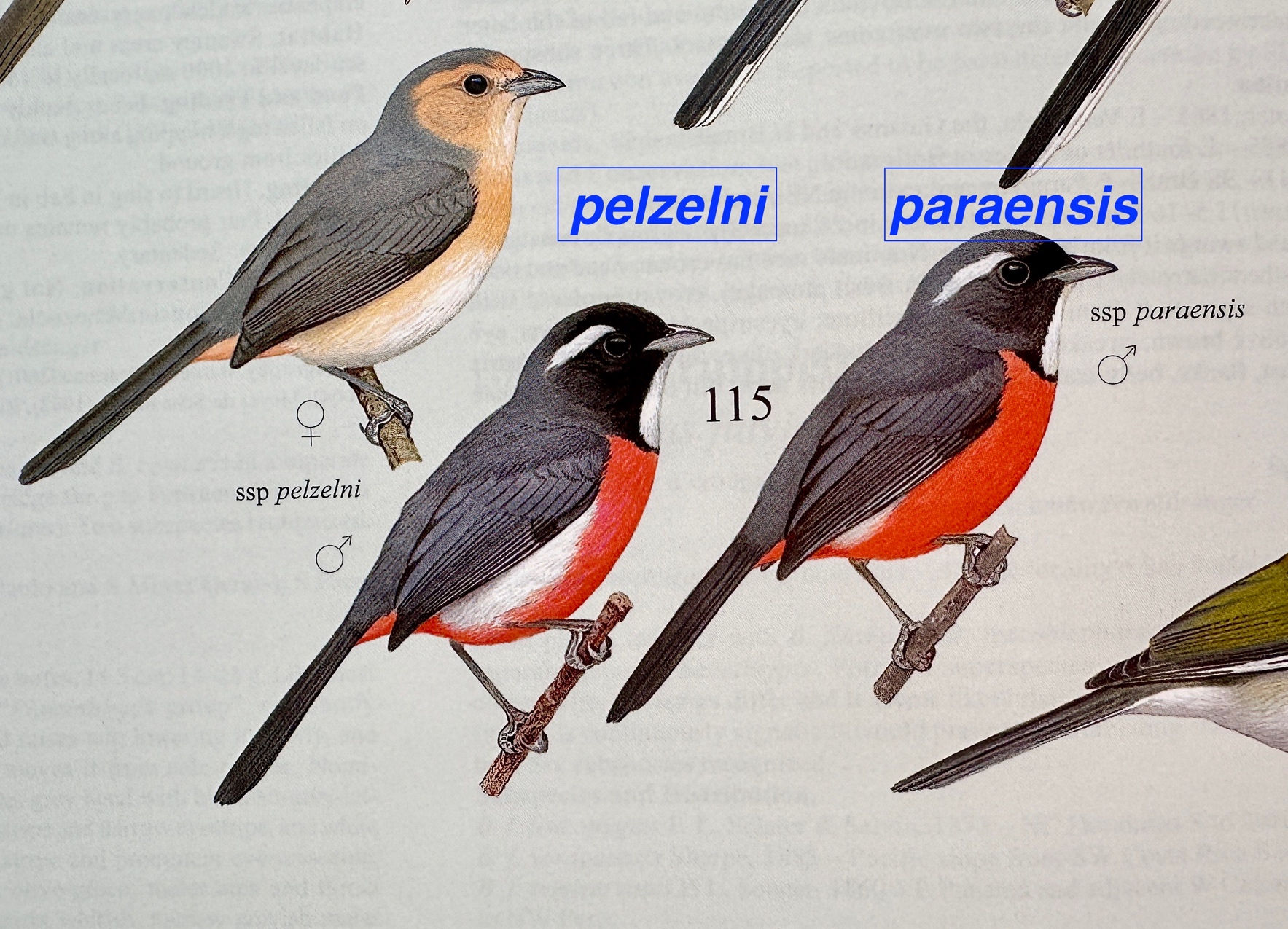
• nominate pelzelni:
largely at the periphery of the Amazon Basin from e. Colombia, c. Venezuela,
the Guianas, ne. Brazil and then S of the Amazon River in central Brazil, W of
the Rio Tocantins, to e. Bolivia
• paraensis: E
of the Rio Tocantins in extreme e. Brazil.
They differ in the
extent of white on the flanks and the amount of black in the face and
crown. Here’s the plate by David Quinn
from HBW:
The peculiar and patchy
geographic distribution is best understood visually – here is the eBird map,
onto which I placed a blue line to roughly represent the Tocantins.
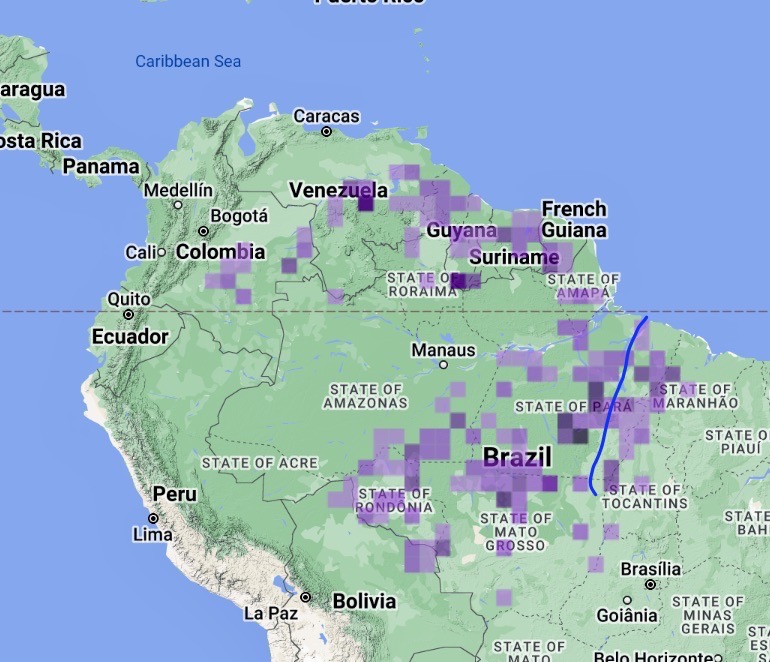
Where
it gets fuzzy for me because I am not familiar with the area is which
localities go with which taxon in the upper Tocantins, Also, the possibility seems strong to me,
again keeping in mind I am unfamiliar with the region, that there could well be
a contact zone in the headwaters of the Tocantins-Araguaia river basin in
southern Tocantins and northern Goias that would be extremely informative.
New
information:
Del
Hoyo & Collar (2014) treated as a separate species based on the Tobias et
al. point scheme as follows:
"Rose-breasted Chat
(Rose-bellied)
“Hitherto
treated as conspecific with G.
pelzelni, but differs in its grey vs black mid-crown and ear-coverts (2);
lack of white flanks (pink extending further onto them or perhaps more grey
extending out from inner flanks) (2); distinctive song, a variable, rather
rhythmic phrase alternating one or more mellow low-pitched down-slurred
whistles and harsher short high-pitched notes, vs a series of repeated rather similar
notes, sometimes ending with a different trilled part or two series of repeated
notes, to be scored as fewer repeated notes (2), shorter song phrases (ns[2]),
different note shapes (ns[1]) and mellow notes having a much narrower frequency
range (2); and possible absence (not yet recorded) of the typical “jrt” call of
G. pelzelni (ns[2])
(2). Monotypic.”
The
vocal data come from Boesman (2016n), who stated:
“A
comparison of song (illustrated with multiple sonograms in the pdf version of
this note): nominate (in the Guianas, N of Amazon), nominate (S of Amazon) and
paraensis (n=4).
“There
is a striking difference in song between paraensis
and nominate! Nominate in all regions (N and S of Amazon) has a song consisting
of a series of repeated notes which are all rather similar-shaped (sometimes
ending with a different trilled part or 2 series of repeated notes). Typically
6-15 repeated notes. Phrase length 1.8 - 3s. Notes have a freq. range of c. 0.7
- 3kHz. Paraensis seemingly
has a song which is a variable, rather rhythmic phrase with alternating a
mellow low-pitched down-slurred whistle (or a short series of these) and
harsher short high-pitched notes. Repeats 1 - 6. Phrase length 0.5 - 1.5s.
Mellow notes have a narrow frequency range of c. 0.7 - 1.1kHz.
The
vocal difference can be scored based on paraensis
having less repeated notes (score 2-3), shorter song phrases (score 2-3) and
different note shapes (1-2) , with mellow notes having a much narrower freq.
range (2), which leads to a total vocal score of about 5. This obviously is
based on only 4 examples of paraensis,
and thus needs further confirmation, but nevertheless seems quite
convincing.
With
just N=4 in Boesman’s analysis of paraensis, I would object in principle
to making any taxonomic conclusion on these putative song differences. In my opinion, Boesman’s “needs further
confirmation” statement is correct. Just
from checking recordings in Macaulay, I noticed this one:
https://macaulaylibrary.org/asset/220845741 (by Ciro Albano
Tocantins, from the Rio Javaés, a right bank tributary of the R. Araguaia,
which itself is a left bank tributary of the Tocantins. This is classic pelzelni song with its
evenly spaced set of upward-inflected notes.
It is west of the Tocantins itself, so if the Tocantins is the barrier,
then it fits that pattern, but the Araguaia is actually bigger than the
Tocantins, so again I want to know (1) is it the Tocantins itself that is the
barrier or the Tocantins drainage? And (2) what did this individual look
like. It is identified as paraensis
in Macaulay, but the basis of this uncertain.
For
reference, here’s one that fits Boesman’s characterization of paraensis
(by Alex Aleixo, Pará at Municipio de Tomé-Açu) from Pará, east of the
Tocantins:
https://macaulaylibrary.org/asset/219437
New
genetic data:
I had originally overlooked the published genetic data on Granatellus in
this region. Alex Aleixo alerted me to
his paper (Dornas et al. 2022), which included the two taxa of Granatellus
in their analysis of genetic variation in the Tocantins drainage. See Areta comments below for full citation
and description of the data There is a
strong genetic break between the two taxa, and the Tocantins is the barrier,
with a 5.3% sequence divergence. But
before anyone gets excited about that, here goes more than my usual diatribe on
genetic distances as a metric for taxon rank.
Below
are comparisons of genetic distance (ND2) between 62 taxon pairs that are in
contact or approach each other in the Rio Branco region studied by Luciano Naka
for his dissertation here at LSU. This
remains as far as I know the largest comparative data set of its kind, and note
that because they were all done by the same methodology using the same genetic
markers on “sedentary” tropical birds, mostly forest-dwellers, this controls
for several potential sources of variation.
I am going to keep using this table every time genetic distance is used
in our assessments of species rank. Note
also the within-family variation – I see no evidence for a phylogenetic
effect. To no one’s surprise, taxa we
rank as species on average show greater between-taxon genetic distance. That is not in dispute. But note that the ranges overlap widely. Just within this sample, if forced to use
genetic distance alone as a metric, any GD measure between 2.0 and 13.0% cannot
be used as a diagnosis for species rank.
What if the sample were expanded beyond sedentary Amazonian birds? Certainly, those ranges would expand in both
directions, but especially downwards, below 2.0%. Some degree of taxonomic circularity
may be biasing this analysis, and Naka et al. rightfully advocated for closer
examination of some of the taxon pairs currently ranked as subspecies. Nevertheless, at this point, the general
signal from genetic distance data is that it cannot be used as a means of
assigning species vs. subspecies rank, as emphasized by Naka et al.
++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++
|
Data from Naka et al., 2012, Am. Nat. 179: E115-E132, Table1) |
|||||||
|
Taxon A |
Taxon B |
rank |
Gdist |
species |
subspecies |
species + SU/SP |
subspecies - SU/SP |
|
Hylophilus ochraceiceps luteifrons |
H. o. ferrugineifrons |
SU/SP |
13.3 |
13.3 |
13.3 |
||
|
Zimmerius acer |
Zimmerius gracilipes |
SP |
12.9 |
12.9 |
12.9 |
||
|
Pheugopedius c. coraya |
P. c. griseipectus |
SU |
12.9 |
12.9 |
12.9 |
||
|
Tyranneutes virescens |
Tyranneutes stolzmanni |
SP |
11.3 |
11.3 |
11.3 |
||
|
Hylophilus muscicapinus |
Hylophilus hypoxanthus |
SP |
11.3 |
11.3 |
11.3 |
||
|
Phaethornis s. superciliosus |
P.s. insolitus |
SU |
10.8 |
10.8 |
10.8 |
||
|
Willisornis p. poecilinotus |
W. p. duidae |
SU |
10.8 |
10.8 |
10.8 |
||
|
Microcerculus b. bambla |
M. b. albigularis-caurensis |
SU |
10.1 |
10.1 |
10.1 |
||
|
Schiffornis t. turdinus |
S. t. amazonus |
SU/SP |
9.7 |
9.7 |
9.7 |
||
|
Myrmotherula guttata |
Myrmotherula hauxwelli |
SP |
11.3 |
11.3 |
11.3 |
||
|
Hypocnemis cantator |
Hypocnemis flavescens |
SP |
9.0 |
9.0 |
9.0 |
||
|
Iodopleura fusca |
Iodopleura isabellae |
SP |
8.8 |
8.8 |
8.8 |
||
|
Glyphorynchus s. spirurus |
G. s. rufigularis |
SU |
8.5 |
8.5 |
8.5 |
||
|
Veniliornis cassini |
Veniliornis affinis |
SP |
8.5 |
8.5 |
8.5 |
||
|
Monasa atra |
M. morphoeus |
SP |
8.5 |
8.5 |
8.5 |
||
|
Onychorhynchus c. coronatus |
O. c. castelnaui |
SU/SP |
8.2 |
8.2 |
8.2 |
||
|
Galbula d. dea |
G. d. brunniceps |
SU |
8.2 |
8.2 |
8.2 |
||
|
Terenotriccus e. erythrurus |
T. e. venezuelensis |
SU |
8.2 |
8.2 |
8.2 |
||
|
Pyrilia caica |
Pyrilia barrabandi |
SP |
7.8 |
7.8 |
7.8 |
||
|
Galbula a, albirostris |
G. a. chalcocephala |
SU |
7.7 |
7.7 |
7.7 |
||
|
Capito niger |
Capito auratus |
SP |
7.7 |
7.7 |
7.7 |
||
|
Topaza pella |
Topaza pyra |
SP |
7.6 |
7.6 |
7.6 |
||
|
Epinecrophylla gutturalis |
E. haematonota |
SP |
7.4 |
7.4 |
7.4 |
||
|
Euphonia cayennensis |
Euphonia rufiventris |
SP |
6.9 |
6.9 |
6.9 |
||
|
Lepidocolaptes a. albolineatus |
L. a. duidae |
SU |
6.9 |
6.9 |
6.9 |
||
|
Dendrocincla m. merula |
D. m. bartletti |
SU |
6.4 |
6.4 |
6.4 |
||
|
Xiphorhynchus guttatus polystictus |
X. g. guttatoides |
SU |
6.4 |
6.4 |
6.4 |
||
|
Xenops minutus ruficaudus |
X. m. remoratus |
SU |
6.3 |
6.3 |
6.3 |
||
|
Dendrocincla f. fuliginosa |
D. f. phaeochroa |
SU |
6.1 |
6.1 |
6.1 |
||
|
Thalurania f. furcata |
T. f. nigrofasciata |
SU |
5.7 |
5.7 |
5.7 |
||
|
Percnostola r. rufifrons |
P. r. minor |
SU |
5.4 |
5.4 |
5.4 |
||
|
Tolmomyias assimilis neglectus |
T. a. examinatus |
SU |
5.3 |
5.3 |
5.3 |
||
|
Xiphorhynchus pardalotus |
Xiphorhynchus ocellatus |
SP |
5.3 |
5.3 |
5.3 |
||
|
Hemithraupis f. flavicollis |
H. f. aurigularis |
SU |
5.0 |
5.0 |
5.0 |
||
|
Thamnophilus amazonicus divaricatus |
T. a. cinereiceps |
SU |
4.3 |
4.3 |
4.3 |
||
|
Automolus infuscatus cervicalis |
A. i. badius |
SU |
3.7 |
3.7 |
3.7 |
||
|
Microbates collaris torquatus |
M. c. collaris |
SU |
3.0 |
3.0 |
3.0 |
||
|
Tolmomyias poliocephalus sclateri-klagesi |
T. p. poliocephalus |
SU |
3.0 |
3.0 |
3.0 |
||
|
Myrmothera c. campanisona |
M. c. dissors |
SU |
2.8 |
2.8 |
2.8 |
||
|
Pteroglossus aracari |
Pteroglossus pluricinctus |
SP |
2.8 |
2.8 |
2.8 |
||
|
Cymbilaimus l. lineatus |
C. I. intermedius |
SU |
2.7 |
2.7 |
2.7 |
||
|
Myrmoborus myotherinus ardesiacus |
M. m. elegans |
SU |
2.7 |
2.7 |
2.7 |
||
|
Momotus m, momota |
M. m. microstephanus |
SU |
2.3 |
2.3 |
2.3 |
||
|
Gymnopithys rufigula |
Gymnopithys leucaspis |
SP |
2.3 |
2.3 |
2.3 |
||
|
Dendrocolaptes c. certhia |
D. c. radiolatus |
SU |
2.1 |
2.1 |
2.1 |
||
|
Brotogeris chrysoptera |
Brotogeris cyanoptera |
SP |
2.0 |
2.0 |
2.0 |
||
|
Piculus f. flavigula |
P. f. magnus |
SU |
2.0 |
2.0 |
2.0 |
||
|
Celeus u. undatus |
Celeus u. grammicus |
SU |
2.0 |
2.0 |
2.0 |
||
|
Thamnophilus murinus cayennensis |
T. m. murinus |
SU |
2.0 |
2.0 |
2.0 |
||
|
Cercomacra cinerascens immaculata |
C. c. cinerascens |
SU |
2.0 |
2.0 |
2.0 |
||
|
Schistocichla l. leucostigma |
S. 1. infuscata |
SU |
1.9 |
1.9 |
1.9 |
||
|
Trogon r. rufus |
T. r. sulphureus |
SU |
1.9 |
1.9 |
1.9 |
||
|
Cercomacra tyrannina saturatior |
C. t. tyrannina |
SU |
1.9 |
1.9 |
1.9 |
||
|
Synallaxis rutilans dissors |
S. r. confinis |
SU |
1.7 |
1.7 |
1.7 |
||
|
Piprites c. chloris |
P. c. tschudii |
SU |
1.7 |
1.7 |
1.7 |
||
|
Sittasomus griseicapillus axillaris |
S. g. amazonus |
SU |
1.6 |
1.6 |
1.6 |
||
|
Tachyphonus s. surinamus |
T. s. brevipes |
SU |
1.5 |
1.5 |
1.5 |
||
|
Pithys a. albifrons |
P. a. peruvianus |
SU |
1.3 |
1.3 |
1.3 |
||
|
Hemitriccus zosterops rothschildi |
H. z. zosterops |
SU |
1.2 |
1.2 |
1.2 |
||
|
Myrmotherula a. axillaris |
M. a. melaena |
SU |
1.2 |
1.2 |
1.2 |
||
|
Celeus t. torquatus |
C. t. occidentalis |
SU |
1.1 |
1.1 |
1.1 |
||
|
Celeus e. elegans |
C. e. jumanus |
SU |
0.8 |
0.8 |
0.8 |
||
|
mean corrected genetic distance |
5.6 |
7.7 |
4.8 |
8.1 |
4.4 |
|
range |
N |
|
|
species (SP) |
2.0-12.9 |
17 |
|
subspecies (SU) |
0.8-13.3 |
45 |
|
species incl. possible splits (SU/SP) |
2.0-13.3 |
20 |
|
subspecies excl. possible splits (SU/SP) |
0.8-12.9 |
42 |
|
SU/SP = taxa currently treated as subspecies but under active
investigation |
||
++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++
In
the case of these two Granatellus taxa, a genetic distance of 5.3% falls
very close the mean genetic distance of all taxa in Naka et al.’s analysis and
cannot be considered, in my opinion, relevant to taxon rank.
Discussion
and Recommendation:
Here
is an expanded view of the David Quinn plate so that you have a broader context
for comparisons of plumage among species in Granatellus:

The
basic plumage pattern in the genus is fairly conservative, with the differences
between paraensis and pelzelni (extent of white on flanks, cheek
color, and superciliary strength) also reflected in differences among the other
two species. As you can see, paraensis
is the extreme in terms of reduction of white flanks but otherwise does not
stand out.
I
don’t have a strong recommendation on this.
I will vote NO for now because of the small N of songs of paraensis
and Boesman’s cautionary statement. The
genetic data certainly suggest a barrier to gene flow. Terra firme taxa ranked as subspecies
frequently show that level of genetic distance across major rivers, although I
did not think G. pelzelni was a species for which a river would be a
barrier, likely because of my ignorance of habitat preference. Regardless, this seems to me to be a case in
which detailed characterization of phenotype distribution in the region of
potential contact is critical, especially towards the upper Tocantins. I suspect that more detailed mapping of vocal
and plumage characters will confirm species rank for these two, but I feel
uneasy about endorsing this without those data.
English
names:
BirdLife International used Rose-bellied Chat for paraensis and retained
parental Rose-breasted Chat for pelzelni s.s. Nominate pelzelni is considerably more
widely distributed, and so retention of the parental name with that taxon is in
line with our SACC guidelines on
English names. The similarity between the names might be
confusing to some but helpful in others in linking the two. The problem is that BOTH taxa have rose
breasts and rose bellies, and so I suspect many people would have a problem
remembering which is which. I suggest
that “Tocantins Chat” has some merit for paraensis because it refers to
its center of distribution in terms of the river and to a lesser extent to the
Brazilian state; also, this part of Amazonia has undergone major deforestation
and so the toponym might have some merit in terms of promoting conservation. On the other hand, if pelzelni
replaces paraensis on the west bank of the Tocantins, the attractiveness
of that name is diminished. In my
opinion, we should do a separate proposal on the English name if the proposal
passes.
References: (see SACC
Bibliography
for standard references)
Boesman, P. (2016). Notes on the vocalizations of
Rose-breasted Chat (Granatellus
pelzelni). HBW Alive Ornithological Note 384. In: Handbook of the
Birds of the World Alive. Lynx Edicions, Barcelona. https://doi.org/10.2173/bow-on.100384
Van Remsen, July 2024
Vote tracking chart:
https://www.museum.lsu.edu/~Remsen/SACCPropChart968-1043.htm
Comments from Areta: “I vote NO to the split.
Although there is a reasonable case for the split based on minor plumage
distinctions and deep mtDNA differentiation, there is conflicting vocal
evidence that I would like to see sorted before changing our taxonomy.
“Dornas
et al. (2022) sequenced 22 pelzelni (including the Guianan population)
and 2 paraensis. Terry Chesser downloaded 4 and 2 sequences and found
that mtDNA distance was 5.3%.
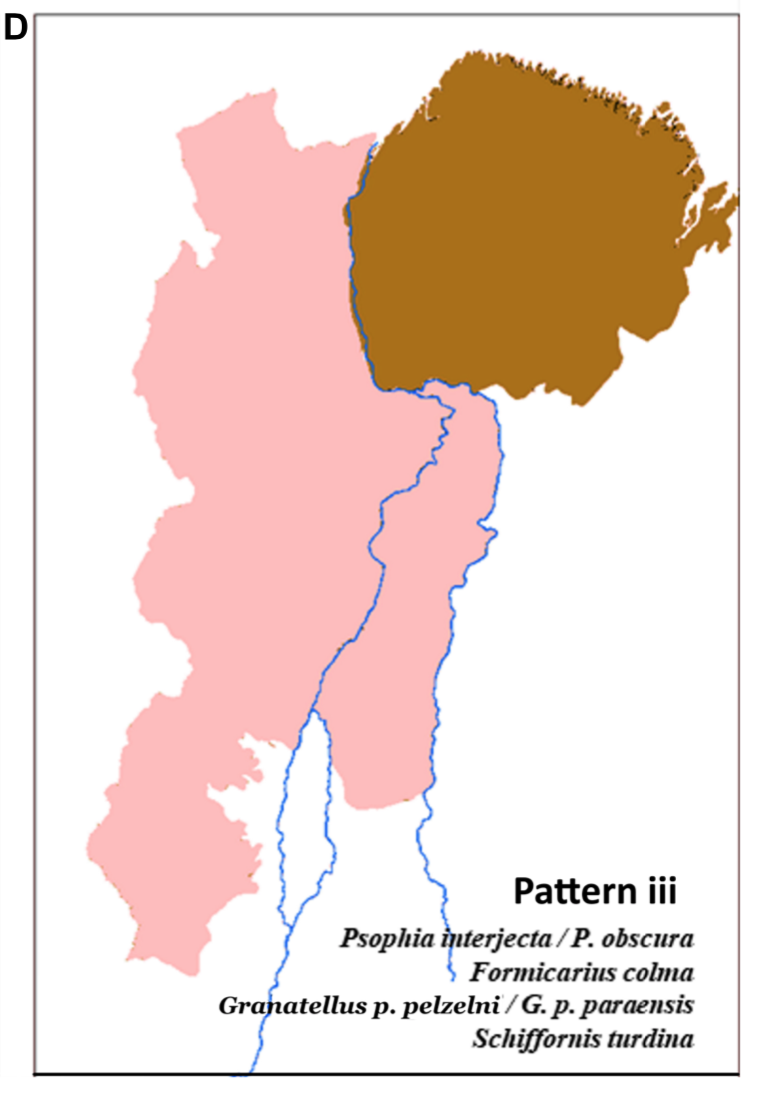
“Here
is Dornas et al´s figure 2D:
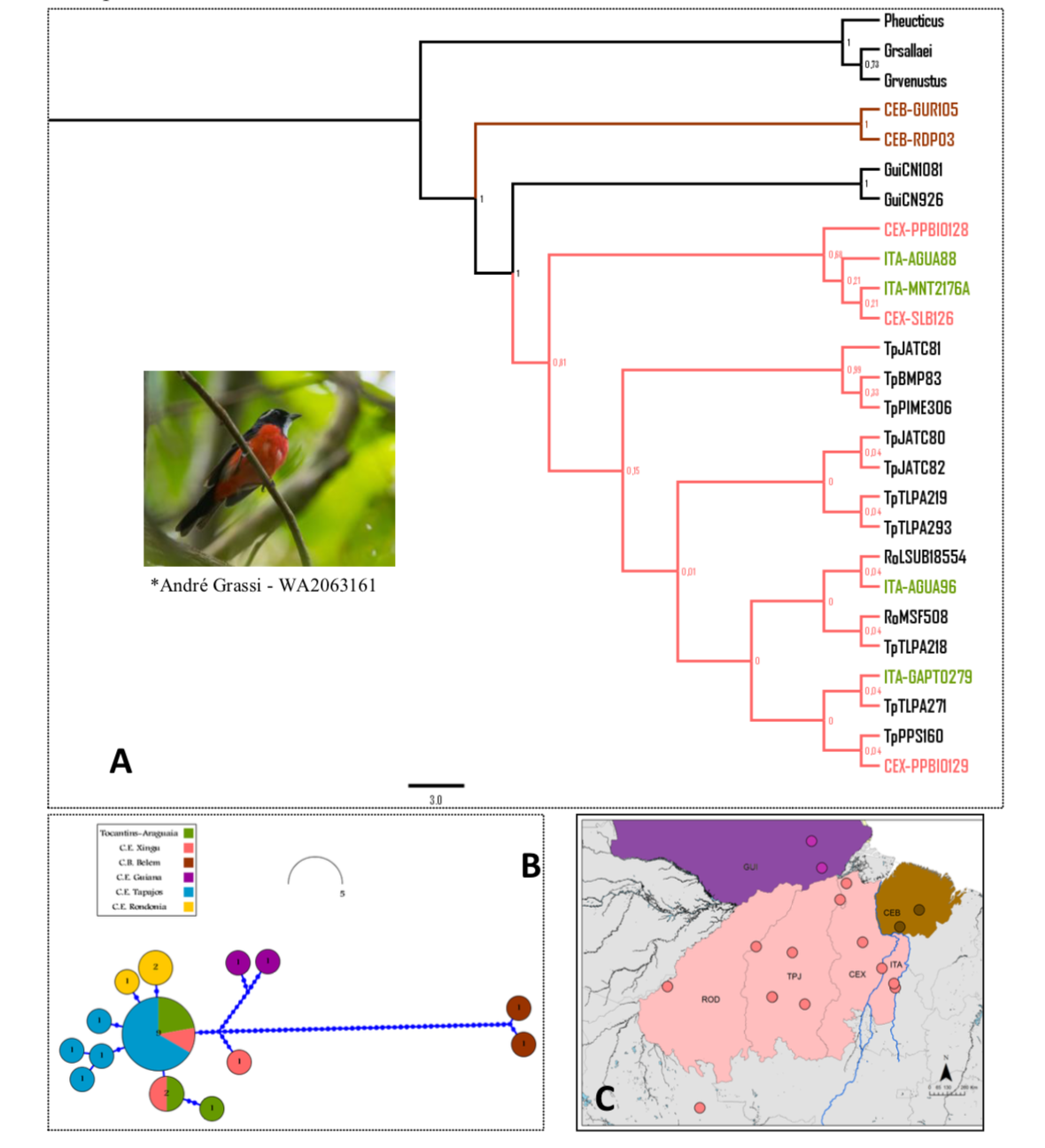
“And
their supplementary Figure S12: ‘Supplementary Figure 12. Phylogenetic tree (A) obtained by
Bayesian Inference (BI) for Granatellus pelzelni accompanied by
the respective haplotype network (B). Dots represent sampling localities within
our target area, with different colors denoting phylogenetic/haplotype
relationships among sampled individuals (C). From 24 sequenced individuals
(Table S2), a total of 14 haplotypes for the mitochondrial marker ND2 in 1033
bp fragments with the following base frequencies were retrieved: A = 0.3035, C
= 0.3643, G = 0.0991, T = 0.2332. As outgroup, sequences from Granatellus
sallaei (GB: EF529922.1), G. venustus (GB: EF529921.1) and
Pheucticus ludovicianus (GB: AF290108.1) were used. A total of 81
sites with nucleotide substitutions were identified. The evolutionary model
selected for BI by JMODELTEST was TRN+I (nst=6, rates=equal, pinvar=0.6100).
CEB = Belém Area of Endemism; CEX = Xingu Area of Endemism; and ITA = Tocantins
Araguaia Interfluve. Populations of Granatellus pelzelni
presented strong phylogeographic structure, with the occurrence of three
independent lineages: G. p. paraensis (BAE), G. p.
pelzelni (TAI and XAE and westward), and a third and apparently unnamed
lineage on the Guiana Area of Endemism (GAE). An asterisk (*) denote the photo
́s authorship along its accession number in the WikiAves (wikiaves.com; WA)
citizen science portal.’ “
Now,
I am confused by some recordings from Maranhão, where supposedly only paraensis
should be present. A recordist attached sex to two birds that were singing
together (and based on his comments, he seems quite familiar with the taxon paraensis,
to the point that he indicates that the females often give this song which
males never do). According to this, then the male paraensis would have a
song that is overall similar in structure to that of pelzelni, and not
radically different as Boesman (2016) indicated. However, I cannot rule out
that the recordings might have been misidentified, as most other recordings of paraensis
do follow Boesman´s description (certainly, all the ones in XC do so, as well
as those by Aleixo in the ML):
Male
https://macaulaylibrary.org/asset/194395401
[sounds quite similar to this one, from Pará,
west of the Tocantins river https://macaulaylibrary.org/asset/375366741]
Female
https://macaulaylibrary.org/asset/194393221
Other recordings from eastern Pará:
Female?
https://macaulaylibrary.org/asset/214418071
Male?
https://macaulaylibrary.org/asset/194197101
“If
this interpretation is correct (I have seen Granatellus pelzelni a few
times, and maybe recorded it in SE Venezuela ages ago, but I do not claim
having a deep knowledge of this taxon), then the vocal differences need to be
reassessed, as clearly it is a weak basis to compare voices of males of one
taxon to females of the other one (although maybe only females of paraensis
give this multi-noted song?).
“The genetic break seems quite deep and abrupt,
but I would like to have more clarity on the situation with vocalizations.
There is a possibility that (vocally) Boesman might have compared pears to
apples. This does not explain the deep genetic break, but (if correct)
seriously undermines the vocal arguments. paraensis and pelzelni are presumably
allo/parapatric, but if one takes Boesman´s analyses as such, then the
recordings I point out above would indicate sympatry (alternatively, one can
interpret that the vocal differences are not as described and that both taxa
have structurally similar songs)..
“Dornas T, Dantas SM, Araújo-Silva LE,
Morais F and Aleixo A (2022) Comparative Phylogeography of Birds Across the
Tocantins–Araguaia Interfluve Reveals a New Biogeographic Suture in the Amazon
Far East. Front. Ecol. Evol. 10:826394. doi: 10.3389/fevo.2022.826394”
Comments
from Robbins:
“NO. Although the mitochondrial data suggest two
species, as Nacho points out there seems to be confusion on vocal comparisons.
The plumage differences may not be significant, note the differences between
nominate Granatellus venustus and G. v. francescae, where the
differences between those two are greater than those between the two pelzelni
taxa.
“Although I'm on the fence on this one, I would like to see
clarification on the vocalizations before voting Yes for recognizing paraensis
as a species.”
Comments
from Stiles:
“NO for now, for about the same reasons as for the Xenodacnis split, we
need a more thorough vocal analysis with definite data for the locations of the
birds recorded for a final decision. The plumage differences, to my eye, are of
the magnitude that better characterizes subspecies rather than species.”
Comments
from Del-Rio:
“YES. 5.3% mitochondrial divergence has to mean
something... I do not oppose "NO" votes... I agree that more data are
needed, but it's quite hard to find this level of mitochondrial divergence in
populations with similar geographic ranges...”
Comments
from Lane:
“NO. From the information provided in others’ comments above, it seems that
separating paraensis from pelzelni is poorly supported by
existing evidence. I would much rather wait for better evidence than act on
what little is available at present.”
Comments
from Claramunt:
YES. The birds look similar, but all Granatellus species look similar.
The fact is that paraensis differs from pelzelni in multiple
plumage traits. Songs seem to differ too, and mtDNA, although the sample size
is small, suggests substantial divergence.”
Comments
from Zimmer:
“NO. I suspect something interesting
might be going on here, given the mitochondrial divergence and the plumage
differences, which, although relatively slight, are about on par with plumage
divergence in this genus, the members of which are mostly just small variations
on a common theme. However, I don’t think we have enough to go on at this point
to draw any definite conclusions.
Reading this Proposal, and listening to the vocal sample (ML219437, from
E of the Tocantins, by Alex Aleixo) that Van included in the Proposal, stirred
something from the distant past in my brain.
In August of 2007, Andy Whittaker and I spent a week surveying birds
based out of a logging camp in the Rio Capím region
(E of the Tocantins). I remembered
encountering Rose-breasted Chat there, and was pretty sure that I had tape-recorded
it. I just went back through my tape
logs from that trip, and a quick perusal turned up only a single recording of Granatellus,
which came at the end of a long sequence that began with Formicivora grisea,
and then switched to various species calling overhead from a mixed-species,
mid-level flock. At some point, with the
tape still running, I switched onto some repeated single-note calls, that may
have been from a Granatellus, but if so, sounded somewhat different from
what I am used to from nominate pelzelni. In any case, I then locked onto several songs
that are almost a perfect match for the Aleixo recording of paraensis,
and very similar to the ones labeled as “Female” and “Female?” that Nacho
shared from eastern Pará. I would
characterize these short, but variable (compared to typical repetitive series
given by nominate pelzelni) songs as suggesting a sweeter sounding
Dusky-capped Greenlet or the phrases of some vireo. Anyway, after recording several of these
songs, there is an obvious break in my recording, without a voice annotation,
followed by a long series of very excited sounding Granetellus
songs that are pretty typical to my ears, of songs given by nominate pelzelni. I recorded the Granatellus right down
to the end of Side B on the cassette, and barely squeezed in an annotation of
“Songs of Granatellus pelzelni, preceded by Formicivora grisea”
before the tape abruptly ran out. In
listening to the recording, it’s pretty obvious to me that I switched off of
the Formicivora and onto the flock passing overhead, when I heard the
initial short, variable, greenlet-like songs, which would have struck me as
unfamiliar. After recording several of
those, there was the quick, but obvious break in the recording, without an
annotation, which suggests that after taping several of the mystery voices, I
tried playback, which resulted in an aggressive and persistent bout of amped-up
songs from the male Granatellus, and those continued to the end of the
tape. I seem to remember having a pair
of Granatellus there, but can’t say for certain. But if the recordist that Nacho quoted is
correct, and that females of paraensis often give the short song, but
that males never do, then I probably recorded spontaneous songs of the female
of the pair, and when I played those back, it elicited the more typical
sounding pelzelni-like songs from the male. All of which means nothing, other than
calling into question Boesman’s (2016) assertion of striking differences in
song between the two taxa. Now, if no
such female song exists in nominate pelzelni (and I certainly can’t
remember ever having heard or taped this song anywhere else), then that does
suggest that there are some vocal differences.
But the fact that the male songs are so similar, at least in my limited
sample, does suggest that we simply don’t know enough about this particular
situation to justify a split.”
Comments
from Bonaccorso:
“NO. Too many unknowns, potential confusions in the analysis of song
differences, not a good understanding of the contact zone.”
Comments
from Luciano Naka (voting for Jaramillo): “NO. In my view, the evidence provided above
remains insufficient to give species status to the two forms of Amazonian Granatellus.
Personally, I don’t think that genetic distance alone is a good metric for
speciation. It rather indicates lack of current gene flow and therefore a
potential road towards speciation. In this case, in order to see if the
speciation process has been “completed enough”, I would like to see a good map
with more specimens and genomic data from the potential area of contact, first
to understand if i) the Tocantins is the actual current biogeographical
barrier, and ii) if there is any evidence of current gene flow across the
region. I found the topic raised by Nacho most interesting, in that males and
females may have different voices, and should not be compared. Given these
uncertainties, I see a NO vote as an incentive for someone to do a detailed
assessment of this case, obtaining more recordings, specimens, and sequences
from this interesting biogeographic region.”