Proposal (1020) to South
American Classification Committee
Treat Pionus
seniloides as a separate species from P. tumultuosus
Note: This is a
high-priority issue for WGAC. We don’t
normally do proposals on data-deficient issues.
Background: Our SACC note on this is as follows:
33. The subspecies seniloides was formerly (e.g.,
Peters 1937, Meyer de Schauensee 1970) considered
a separate species ("White-capped Parrot") from Pionus tumultuosus
(“Plum-crowned Parrot”), but see O'Neill & Parker (1977), who noted
that the only differences between the two are the degree of saturation of rosy
pigment; this treatment was followed by Collar (1997) and Dickinson (2003), but
not by Forshaw (1989), Fjeldså & Krabbe (1990), or Ridgely et al. (2001).
There is no evidence of intergradation between the two. SACC proposal to treat seniloides
as a species did not pass.
Recent genetic data (Ribas et al. 2007, Smith et al. 2024) indicate that the
genetic distance between them is about the same as other taxa ranked as species
in Pionus. Del Hoyo & Collar (2014) treated seniloides as a separate species. English
name "Speckle-faced Parrot" for composite species follows suggestion
by Fjeldså & Krabbe (1990).
Here
is the original SACC proposal, from 19 years and 844 proposals ago, which was
rejected 3-6 (Schulenberg did not vote):
Proposal
(176) to South American Classification
Committee
Split
Pionus seniloides from Pionus tumultuosus
Background. These two taxa were treated as distinct
species by Peters (1937). Given that Peterson generally lumped on a massive
scale, this is surprising. O'Neill & Parker (1977) noted that the two taxa
appeared to differ only in degree of saturation of the reddish pigment, a
typical mode of differentiation among New World parrots. However, they did not
formally propose to lump the two taxa.
This step was taken by
Collar (1997:463), who stated: "Race seniloides often regarded as a
separate species, but differences between them slight and superficial,
appearing more appropriate for subspecific separation." Dickinson (2003:
202) also lumped them, citing only O'Neill & Parker (1977) in footnote 4.
On the other hand, Forshaw
(1978:524-25) and Fjeldså & Krabbe (1990:214-215) kept the two taxa as
distinct species, the latter authors stating: "A more detailed mapping of
distribution and variation n[orth] of the Carpish Mtns. is needed in order to
state whether this taxon intergrades with the White-capped P[arrot], and should
in fact include it as ssp." Ridgley & Greenfield (2001: 288-89) also
kept P. seniloides as a distinct species, although they stated that
if intergradation between the two taxa occurred in n. Peru, this would justify
lumping them.
The fact remains that there
are no known intergrades between the two taxa. Furthermore, there is no
gradation within each taxon: that is, it is not the case that birds close to
the range of the other taxon approach the other taxon more in coloring than do
birds at the other end of the taxon's range, as might be expected if we are
dealing with subspecies.
In the circumstances, I
consider that the decision to lump the two taxa was premature and that they are
better retained as distinct species.
I also note that Chrysotis
albifrons Bonaparte, 1845, Atti di Società Italiana di Scienze Naturale
e museo civico de Storia Naturale, Milano, 6, p. 404. (Colombia) is an earlier name applying to P.
seniloides. Salvadori (1891: 330) lists Bonaparte's 1845 name in the
synonymy of Pionus seniloides with (nec Sparrm.). However, Psittacus
albifrons Sparrmann, 1788, the basis of Amazona albifrons, does
not preoccupy Bonaparte's name, either by primary or secondary homonymy. To
preserve stability, it is necessary to declare Chrysotis albifrons Bonaparte,
1845 a Nomen oblitum and Psittacus seniloides Massena &
Souancé,1854 a Nomen protectum under ICZN 23.9.1-2 (1999: 28).
RECOMMENDATION
That Pionus seniloides (Massena
& Souancé,1854) and Pionus tumultuosus (Tschudi, 1844) be treated as
distinct species.
REFERENCES:
Collar, N. J. (1997), Family
PSITTACIDAE(Parrots), Pp.280-479 in del Hoyo, J., Elliott, A. & Sargatal,
J. (eds.), Handbook of the Birds of the World, 4, Lynx Edicions,
Barcelona.
Dickinson, E. C. (ed.)
(2003) The Howard and Moore Complete Checklist of the Birds of the
World. Revised and enlarged 3rd edn, Christopher Helm, London.
Fjeldså, J. & Krabbe, N.
(1990) Birds of the High Andes. Zoological Museum, University of
Copenhagen, Copenhagen.
Forshaw, J. (1978), Parrots
of the World, 2nd edn., Lansdowne Press, Melbourne.
O'Neill, J. P. & Parker,
T. A. III (1977), Taxonomy and range of Pionus seniloides in
Peru. Condor 79, (2), 274.
Ridgley, R. S. &
Greenfield, P. J. (2001) The Birds of Ecuador: Status, Distribution and
Taxonomy. Cornell University Press, Ithaca, N. Y.
Salvadori, T. (1891) Catalogue
of the Birds in the British Museum, vol. 20, Psittaci, Trustees of the
British Museum (Natural History), London.
John
Penhallurick, May 2005
_______________________________________________________________________________________
Comments from Zimmer: "YES. Although
the morphological differences between these taxa are not particularly
impressive, the absence of any real evidence of intergradation, combined with
an absence of any published analysis to support the lump is enough to sway me,
at least until such time as evidence to the contrary is published."
Comments from Robbins: "NO. Given that our
current treatment has seniloides as a subspecies, and we have no
new information, I vote "no" until the time comes that we have
information that can guide us into making a more informed decision."
Comments from Stotz: "NO. Whether seniloides
and tumultuous should be lumped or split is an open question.
However, this proposal makes several comments that are erroneous in making it
seem like this is a recent change. It says "However, they [O'Neill and
Parker 1977] did not formally propose to lump the two taxa." In fact, they
did formally suggest that the two taxa be lumped, saying 'We believe, in the
absence of evidence to the contrary, that the two forms of Pionus under
discussion do not differ enough morphologically or ecologically to warrant
being retained as separate species.' It can't get much clearer than that. This
treatment was adopted by Hilty and Brown in 1986, so it didn't have to wait for
Collar to be adopted. I doubt that Forshaw 1978 had seen the 1977 paper, but I
don't have it in order to check.
"In terms of the lack
of intergradation, I would just note that there is a gap of 150 km between the
northernmost tumultuosus and the southernmost seniloides, so the
lack of known intergrades does not in itself suggest anything to me. I have to
agree with Mark on this one. In the absence of new information, I don't see a
good argument for upsetting our status quo."
Comments from Silva: "NO. We need more
information about the putative contact zone between these two taxa."
Comments from Jaramillo: "NO - In the absence
of a new analysis, keep them as they are. Although my hunch tells me that there
are two species here, the lack of information from the intervening zone is a
key missing piece of data."
Comments from Nores: "YES. Aunque las diferencias en plumaje no son tan
grandes, son aparentemente suficientes como para separarlos. La única contra
que yo veo es lo sugerido por Ridgely y Greenfield de que hubo intergradación
en el N de Perú. Si fuera así, habría que juntarlas.”
Here’s
the basic set up. These are two Andean
cloud-forest taxa that are broadly parapatric with the break not at the N.
Peruvian Low/Marañon as usual but rather somewhere in central Peru. Seniloides occurs from nw. Venezuela
and Colombia S through Ecuador to central Peru in dpto. La Libertad, and then tumultuosus
takes over from dpto. Huánuco south through Peru to central Bolivia in dpto.
Santa Cruz. Although usually presented
as abutting in ranges, in fact there is a substantial gap of roughly 150 km <fact check>
between the southernmost record of seniloides and in La Libertad and the
northernmost record of tumultuosus in Huánuco that is perhaps the
largest true sampling gap in Andean cloud-forest birds, at least in Peru – that
gap is completely unexplored, ornithologically, and there are no known barriers
to dispersal in that gap, i.e. as far as we know continuous humid forest. Thus, technically, that gap should appear in
range maps of every Andean forest bird, but I think it’s fair to assume that if
a species occurs on both sides of the gap, the gap is a sampling artifact.
Whether
there is an actual contact zone or a real gap is technically unknown. However, it seems highly improbable that the
only area these widespread Andean Pionus would find unsuitable from Venezuela
to Bolivia just so happens to coincide with the unsampled gap in central Peru. Further, these canopy-dwelling highly volant
taxa would be among the least likely Andean birds to be thwarted by a forested
gap. Keep in mind there is no known
phenotypic variation in this complex across the biggest barrier to dispersal of
Andean forest birds: the NPL/Marañon. I
predict that there is a contact zone in there that will reveal how these two
interact. The SACC note covers most of
the history of their taxonomy.
Here
are some photos from Macaulay Library (top or left seniloides by
Francesco Verenesi from Napo, EC, and bottom or left by Michael Buckham from
Cuzco):


Here
are two plates by F. Jutglar, the one on the left from HBW and the one on the
right in Del Hoyo & Collar (2014):

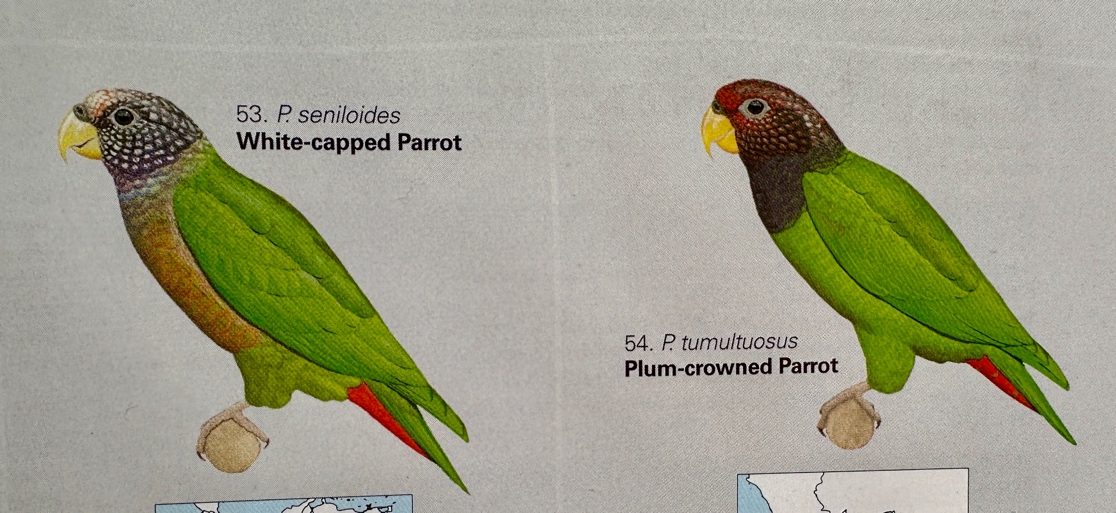
Here
is the plate by Hilary Burn from Schulenberg et al. (2007, Birds of Peru):

Here
are some photos of LSUMZ specimens from Peru.
First our northernmost (n. Ecuador) and southernmost (Cochabamba) specimens:


At
this point, all I want you to note is that the differences between them shown
in the plates are somewhat exaggerated, especially with respect to color intensity. Some of this is likely due to multiple
reproductions of reproductions of the plates, not the artists themselves (for
whom I have the utmost respect for their talents and the challenges in
representing a species in one illustration, particularly when in many cases the
species is unfamiliar to them in life).
Also, at this point one can see why if one were shown only the dorsal
views of the two, one would wonder why anyone would consider them conspecific,
but much less so from the lateral view.
These are the two specimens in our LSUMZ synoptic series, likely chosen
because they represent the extremes in difference between the two taxa: our
northernmost specimen is our whitest, and our Cochabamba specimen is our reddest. That the extremes in color are also the
extremes in geographic representation may just be coincidence, and needs to be
looked at with a larger series.
To
illustrate variation in underparts, here’s the LSUMZ series of seniloides. I don’t have time to sort this out by age,
sex, or molt condition vs. individual variation – the point is only that there
IS substantial variation that needs to be sorted out eventually:

Next,
here are the LSUMZ series of both to show crown color.
1. seniloides, from L to
R: two from La Libertad, then 5 from San Martín and Amazonas, and the in the
separate photo, three from Cajamarca.
Note the variation in extent of white, and if you zoom in, erratic areas
of rose color:


2. tumultuosus, L to R: 1 from
Cochabamba, 2 from Puno, 1 from Junín, 3 from Huánuco. Not as much variation as in seniloides, but
note that the darkest one is the once from Cochabamba and the palest is one of
the three from Huánuco. Somewhat suspicious,
and at least worthy of further investigation, at least if a larger N could be
assembled:

Concerning
the red in the crowns of some seniloides, here are close-ups of the two
with the most red:


Note
that the distribution of reddish color is irregular. This may just be individual or age variation,
analogous to the variation in red feathering in the face of some other New
World parrots, but a hypothesis that that it represents gene flow, past or
present, from southern tumultuosus is worth considering. The instinct of a previous generation of
ornithologists, who typically based their taxonomic conclusions primarily on
phenotype distribution in geographic arrangements of specimens like this, would
be to label this variability as “character instability”, something often
associated with zones of introgression. However,
I didn’t have to go any further than Restall et al. (2006) to see that this
variation occurs in the northern Andes as well:

Although
the basis for determining older vs. younger adults was not given, this
minimally establishes that the occasional red feathering is not concentrated
near the potential contact zone and is unlikely to be due to ongoing gene flow.
Here
is the crown of another specimen of tumultuosus that might have caught your eye
in the photos above, one from Cajamarca:

My
first reaction was naturally that this was going to be what younger individuals
looked like in terms of crown color.
However, the specimen label reads, “left testis 8x5 mm; no bursa”, so
there is no internal sign of this being a young bird. Note also that it has an above average amount
of red in the forecrown. I’m not sure
what my point is here is except to point out additional variation
The
first to propose that seniloides and tumultuosus were conspecific
were O’Neill & Parker (1977), who were reporting some of the first records
of both taxa from Peru. They had few
specimens of either to examine. This
species is not common in collections – collecting canopy parrots in Andean
cloud-forest is not easy. Here is their
rationale:
“After
careful scrutiny of the specimens available to us we have come to the
conclusion that the only difference between P. seniloides and P.
tumultuosus is the amount of rose or plum color present in the plumage of
the head and belly. The northern birds, “P. seniloides,” have only a
wash of this color present on their otherwise whitish head plumage and have the
belly with a variable amount of rosy color, but the southern birds. “P.
tumultuosus.” have the head strongly marked with this color and have solid green
bellies. The older birds of either form apparently have the greatest saturation
of the rosy coloration in their plumage.”
And
“We
believe, in the absence of evidence to the contrary, that the two forms of Pionus
under discussion do not differ enough morphologically or ecologically to
warrant their being retained as separate species.”
Some
but by no means all subsequent classifications treated them as
conspecific. Schulenberg et al. (2007;
O’Neill and Parker were co-authors, the latter posthumously) maintained then as
conspecific. No differences in vocalizations
were mentioned.
New
information:
Del
Hoyo & Collar (2014) treated seniloides as a separate species based
on the Tobias et al. point scheme as follows:
earlier
information:
“Often treated as a distinct species, but
united with P. tumultuosus (e.g. in HBW) following
“(1). Here reseparated on account of its
white forehead and grey-scaled white crown vs all plum-red crown (3); white and
grey vs plum-red, white and blackish flecks on face, ear-coverts, chin and
throat (2); brownish-grey extending down breast to belly on typical birds vs
shading rapidly to green on mid-breast (2). Monotypic.”
There’s
not really any new information here, just plugging known plumage characters
into a controversial methodology. Note
that the threshold of 7 points achieved solely on the basis of plumage
characters. Pionus and many other
parrot genera have complex, gaudy plumages, so the chances of any taxa reaching
the threshold of 7 are obviously much greater than in other groups of birds
with more conservative plumage evolution, including other genera of parrots. This is one of the fundamental conceptual
flaws in the Tobias et al. scheme, i.e. no consideration of phylogenetic biases
in plumage variation.
As
a reminder, the Tobias et al scheme is based on scoring 58 pairs of parapatric
bird taxa for which fundamental details of their interactions. However, of those 58, 55 (95%) are
passerines, and these are almost all either temperate latitude passerines or
thamnophilids, i.e. even within passerines, the foundation of the system is
highly non-random. How many pairs of
parrot taxa went into the Tobias scheme on which this split is based? ZERO.
For a full critique of using the Tobias et al. scheme for changes in
taxonomic rank, see my two reviews (Remsen 2015, 2016).
The only truly new data are the genomic data of
Ribas et al. (2007) and Smith et al. (2024), which confirm that they are sister
taxa and show that they differ genetically, neither of which is of course a
surprise to anyone. Here is a screen
shot of the relevant part of the tree from Smith et al. (2024). The degree of differences is toward the low
end of that shown by taxa traditionally treated as separate species, but
without deeper sampling at the subspecies level, there are insufficient data
for a comparative analysis, and in in my opinion the genetic data are
inconclusive. Here is the relevant
portion of the Smith et al. tree:
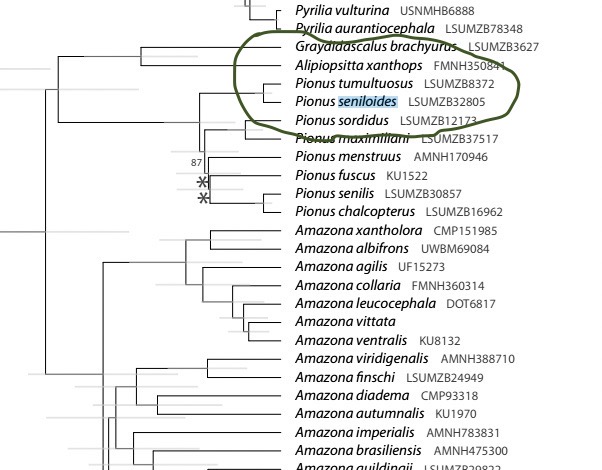
Discussion
and Recommendation:
This
is another difficult situation to assess.
Reasons
to vote YES for the split could be: (1) Those differences in head and breast
color are substantial, arguably at the subjective limits of variation
traditionally relegated to subspecies rank, and presumably are quite striking
to these highly social birds. (2) There is no sign of consistent geographic
variation over the large ranges of the two taxa, and thus if there is a hybrid
zone between the two, it must be entirely within the unsampled gap, without any
known signs of gene flow at their nearest sampling points.. (1+2+3) Therefore,
burden of proof rests on treatment as conspecific, especially since the paper
that proposed treating them as conspecific was not quantitative.
Reasons
to vote NO could be: (1) Taxa treated as separate species usually have
differences in flight calls. One
exception to this is Pionites melanocephalus/P. leucogaster , but that
is also a controversial case in terms of species limits. (2) Are there really
enough specimens and have they been examined sufficiently thoroughly to really
be sure there is no gene flow?
Certainly, this level of study of geographic in phenotypic would be
required for a modern study. (3) Eventually, the sampling gap is going to be
filled, so what’s the rush in pushing a change at this point before we have a
chance to get a solid answer on what’s going on at contact?
I’m
going to vote NO on this one, but without strong conviction. To be convinced to split them, I’d like to
see a synthesis of comparisons of similar cases in parrot genera in terms of
what are traditionally treated as sufficient differences in plumage data for
treatment as separate species. Better
yet, a synthesis of “knowns”, i.e. what’s known about plumage differences and
contact zones in parapatric parrot taxa treated as species and subspecies. Unfortunately, I think the number of
well-studied cases in parrots is too few to provide much guidance. Another approach would be to synthesize the
degree of plumage differences in sympatric congeners in parrots, i.e. cases in
which the taxonomy is indisputable – are the differences here similar to those
shown by sympatric congeners? None of
these approaches would be very satisfying, but at least they would provide a slightly
better foundation for a temporary solution until the contact zone is studied.
English
names:
The pre-lump names for the two were White-capped Parrot and Plum-crowned
Parrot, both good names, so that’s what our SACC names would be unless someone
wants to do a proposal.
References: (see SACC
Bibliography
for standard references)
O'NEILL, J. P., AND T. A. PARKER III.
1977. Taxonomy and range of Pionus "seniloides"
in Peru. Condor 79: 274.
Van Remsen, July 2024
Vote tracking chart:
https://www.museum.lsu.edu/~Remsen/SACCPropChart968-1043.htm
Comments
from Areta: “NO. I vote to follow
O´Neill & Parker in considering seniloides
and tumultuosus as
part of a single species. I would like to see better DNA evidence and at least
a vocal analysis and a careful analysis of plumage variation across geography.
These analyses could provide support one way or the other, but without them, I
prefer to follow those who have dedicated more field effort. (i.e., no split).
Van´s sample of specimens made me raise my eyebrows, even when anyone who had
looked carefully enough at Pionus
knows about the sheer amount of variation that they exhibit. Learning about how
the parrots behave in their contact zone will be key. Meanwhile, the large
amount of variation coupled to a reduced degree of genetic differentiation call
for caution.”
Comments
from Stiles:
“NO for now (again). The decisive data for resolving this one will have to come
from specimens with associated vocal and genetic information, collected in that
150 km “contact”(?) zone.”
Comments
from Del-Rio:
“NO. After studying pigmentation more closely, I am
starting to believe these changes in carotenoid based pigments are highly
plastic and dependent on food availability. I also think the putative contact
zones should be better sampled.”
Comments
from Lane:
“NO. For now, I would stay with the decision made by O’Neill and Parker (1977).
Until we have a better understanding of what’s going on in central Peru, it
seems ill-advised to jump to conclusions in the absence of evidence.”
Comments
from Robbins:
“More information is needed, as pointed out in the
proposal and by Nacho, thus for now I vote NO.”
Comments
from Claramunt:
“YES. I vote to revert to the two-species scheme. A “belief” that two taxa do
not “differ enough” is not acceptable evidence of conspecificity. There is no
evidence of intergradation despite potential contact. Even if they were
allopatric, what is the logic, under the biological species concept, of lumping
to taxa that differ so much in a trait that with obvious potential influence in
species recognition and sexual selection? Most parrot species that differ that
much in their head and face color are treated as different species.”
Additional
comments from Stiles:
“Santiago´s point that the two taxa are as different in plumages as are several
other pairs of species in Pionus is well taken, but in at least
some such cases, differences in ecology, voice and/or genetics also occur. The
plumage differences could be the result of only 1 or 2 mutational differences
in the synthesis and deposition of red pigments. Here, genetic data are
insufficient for a clear verdict and no good vocal information appears to
exist. Are the plumage differences sufficient to conclude that were they to
come into contact, they would not interbreed – or conversely, be genetically
compatible enough that gene exchange could occur? We don’t know, and won’t know
enough to answer these questions until we have specimens (and, hopefully,
recordings) from that frustrating 150 km gap. Until then, the current consensus
considers these taxa as subspecies, and the only advantage to calling them
species (with an equal lack of evidence) would be better to call attention to
and maintain more interest in this problem. If only for this rather quixotic
reason, I could be persuaded to vote Yes to the split.”
Additional
comments from Remsen:
“I think Santiago is probably correct on this, but I just can’t bring myself to
switch to a Yes vote on this until we have more data, either on the sampling
gap or flight call differences. I’m
always queasy about using plumage variation in parrots as markers for species
limits given how much individual variation exists, as in this case.”