Proposal (1023) to South
American Classification Committee
Recognize
Trichothraupis griseonota as a
separate species from T. melanops
Our
current SACC note reads:
"14f. The Andean population of Trichothraupis
melanops was described as a new species, Trichothraupis griseonota,
by Cavarzere et al. (2024). SACC proposal badly needed."
Cavarzere
et al. (2024) described the Andean-foothill populations of T. melanops as a new taxon at the species level under the name T. griseonota, following the PSC (but
arguing that the same interpretation would be possible under the BSC). They
based their conclusions on the examination (and measurement) of 314 specimens from the Atlantic Forest population, and 52
from the Andean-foothill population. They also examined 520 sound recordings of
the Atlantic Forest population, and 19 from the Andean-foothill population.
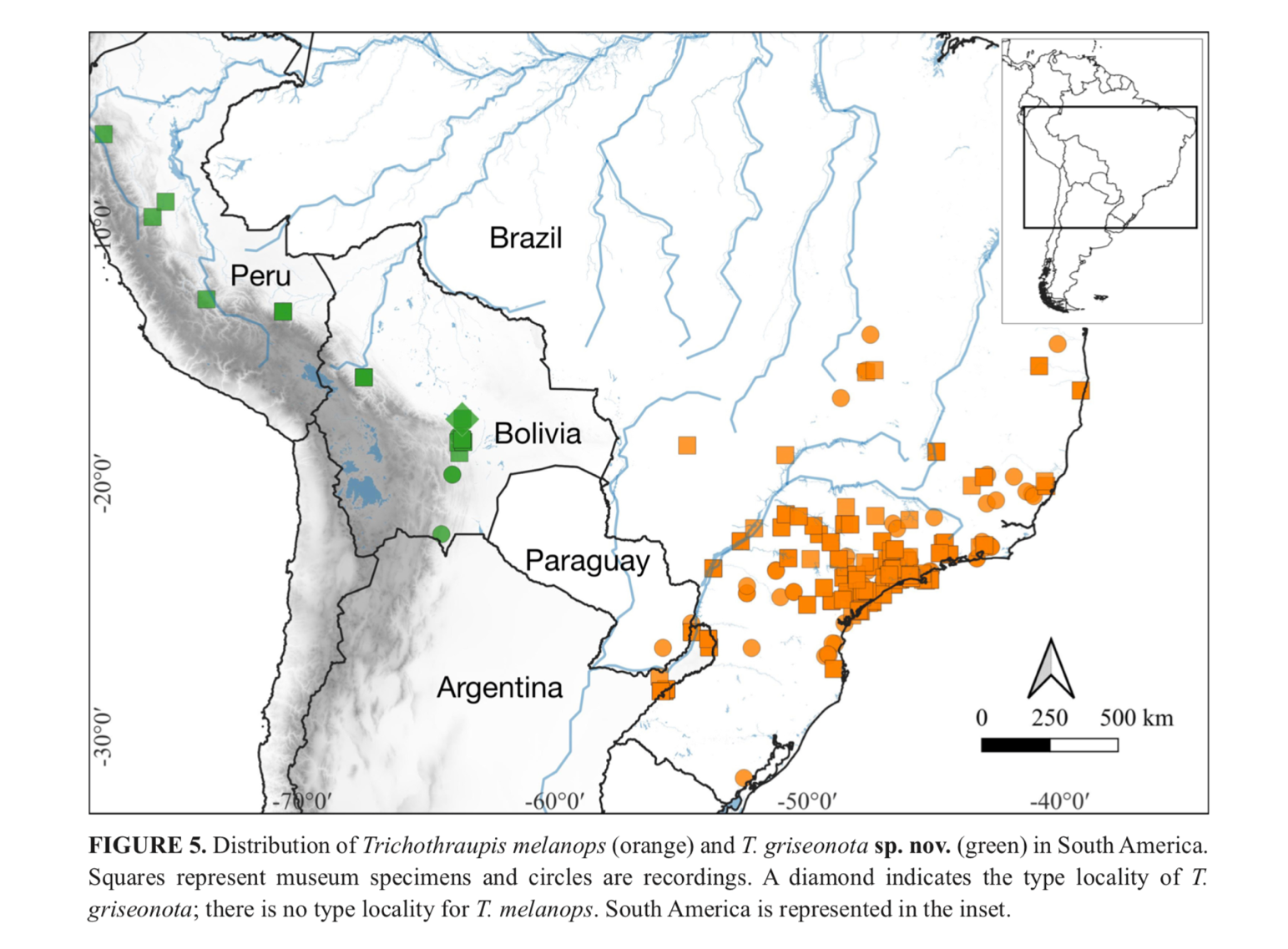
The current distribution of both taxa is more extensive than shown
in their specimen map (Figure 5, below; e.g., there are overlooked documented
photographic, sound, and published specimen records in NW Argentina,
photographic records in the tall, riverine forests in eastern Formosa in NE
Argentina, and numerous records along the Paraguay river).
Earlier,
Zimmer (1947; note number 51, and not 52 as cited in Cavarzere et al.) referred
to geographic variation in Trichothraupis
stating that
"There is a slight possibility that distinctions might
be found in Peruvian specimens as compared with Paraguayan and Brazilian
material, but the range of variation in the species is so great (126 specimens
examined) that little is to be expected. Two adult males from Bolivia (Province
of Sara) have a greater posterior extension of the black area on the sides of
the face than any other males at hand from any locality, but there is
considerable variation within this extreme limit. These two males also have the
longer upper tail-coverts decidedly blackish, with narrow olive tips, but this
character is present in a small number of Brazilian and Argentine birds, also,
and is of doubtful taxonomic value.”
Prior
to this, Hellmayr (1936) stated in a footnote:
"The
supposed distinction of a northern form (auricapilla),
which we at one time advocated, has not been corroborated by additional
material since. Birds from Espirito Santo and Rio de Janeiro seem to be
precisely like others taken in southern Brazil and Paraguay. Bolivian and
Peruvian specimens merely differ by very slightly paler under parts, but the
divergency appears to me too insignificant to justify its recognition in
nomenclature, although there is obviously a wide gap between the eastern and
the Andean ranges of the species."
Therefore
both, Zimmer and Hellmayr discussed slight plumage variation in T. melanops.
The holotype of griseonota
is "MACN 8979a, Male. BOLIVIA, Buenavista, Santa Cruz, 30 July 1916, J.
Steinbach leg., 450 m". So, how is it possible that the holotype is
not shown in the paper itself or in the supplementary material? It is not too much
asking to show photographs of a holotype in the 21st century!
Cavarzere et al. (2024) provide the
following diagnosis:
"Diagnosis. Four fixed plumage traits and one
morphometric trait diagnose Trichothraupis griseonota from its sister
species, T. melanops. The first and most noteworthy is the black facial
mask. In the new species, it includes the auricular region (Fig. 1; Fig. S3),
whereas in T. melanops this mask is only a narrow line behind the eye,
not reaching the auriculars. In a few Atlantic specimens (AMNH 774505, LSUMZ
59405, MZUSP 27851) there is some black in the auriculars, but in those cases,
it is mixed with green, producing a mottled appearance, unlike the homogeneous
black in T. griseonota. A second diagnostic character is the paler
underparts of adult males and females. This is especially evident in the
undertail coverts, which are a cream color to buff-yellow in T. griseonota,
versus cinnamon in T. melanops, and in the chest, in which both species
have a more orange tone than in the belly, but less distinctly so in the new
species, where the color of the chest is more buff-yellow, versus buff in T.
melanops (Fig. 1). Adult females of T. griseonota are also somewhat
paler in the underparts than in T. melanops, but the difference is not
as pronounced as in males, and sexual dimorphism of plumage color in the
underparts is more evident in T. griseonota than in T. melanops.
The third plumage characteristic is the color of the back of adult males, which
is greyer in T. griseonota, versus being more greenish-olive in T.
melanops (Fig. 1; Fig. S3). The last character in which the two species
differ is the breast coloration of females, which is subtly but consistently
darker in Atlantic populations (Fig. 2). Trichothraupis griseonota also
has significantly shorter tarsi compared to T. melanops (Fig. 4)."
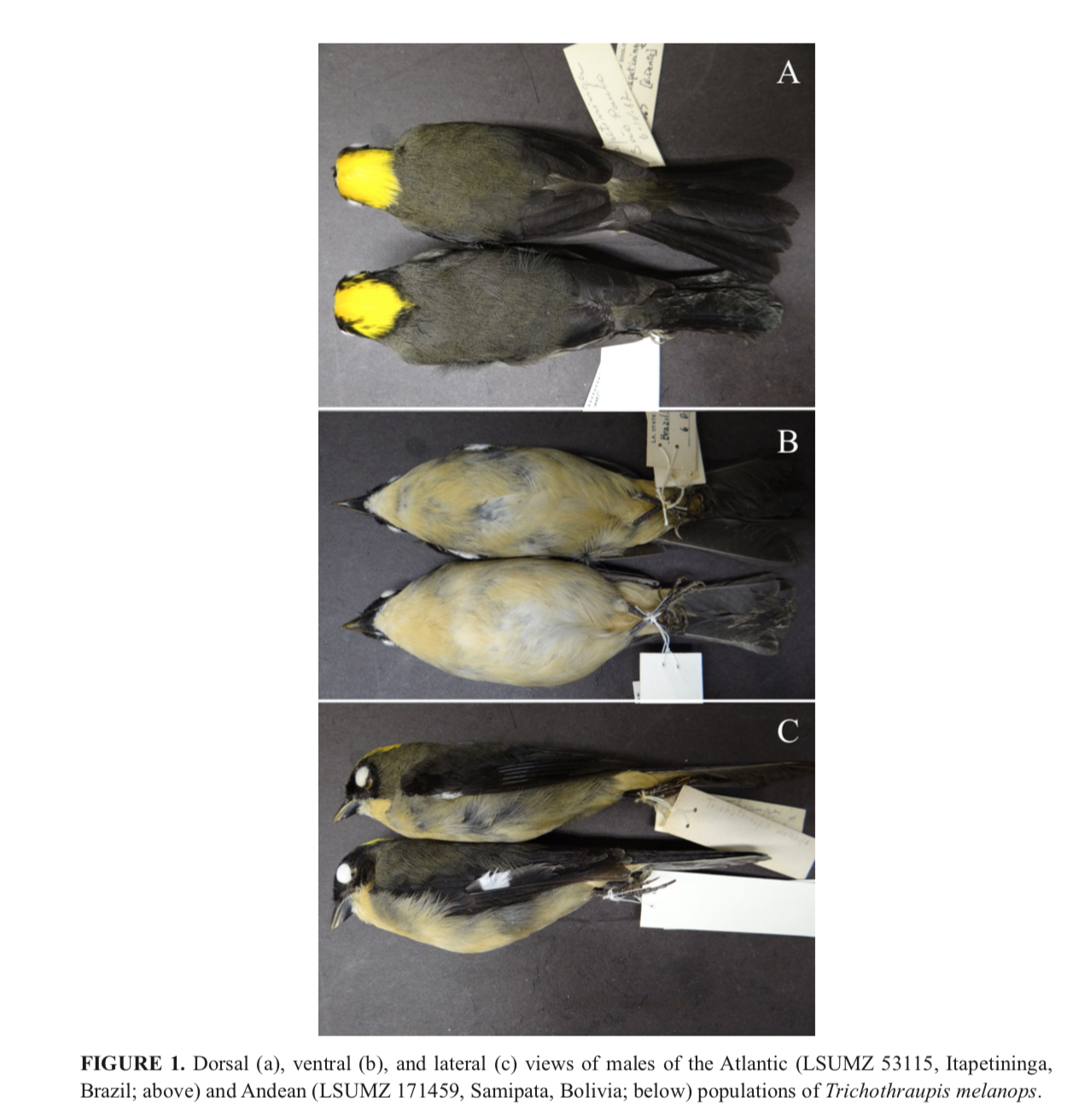

Looking at these photographs we see
minimal plumage differences showing very slight differences in dorsal
coloration, even more subtle differences in ventral coloration, and a
noticeable (yet variable) difference in the amount of black in the goggle.
"Morphometrics.
The Andean population has
significantly shorter tarsi, on average, than the Atlantic population, but all
traits show measurement overlap. The mean measurements of both populations are
shown in Table 1. Principal Component Analysis indicates that morphometrics
segregate the two populations, although incompletely (Fig. 3). Within this
context, the two-factor comparison of sex and populations only showed
significant differences for culmen (p<0.010) and wing (p=0.000) measurements
between sexes, while tarsi measurements (p=0.000) were significantly shorter
among Andean populations (Table 1). There were no significant differences in
the interactions between them (Bonferroni post hoc test p>0.02). Therefore,
the Andean population is morphologically distinguished based on plumage color
patterns and tarsus length (Table 2)."
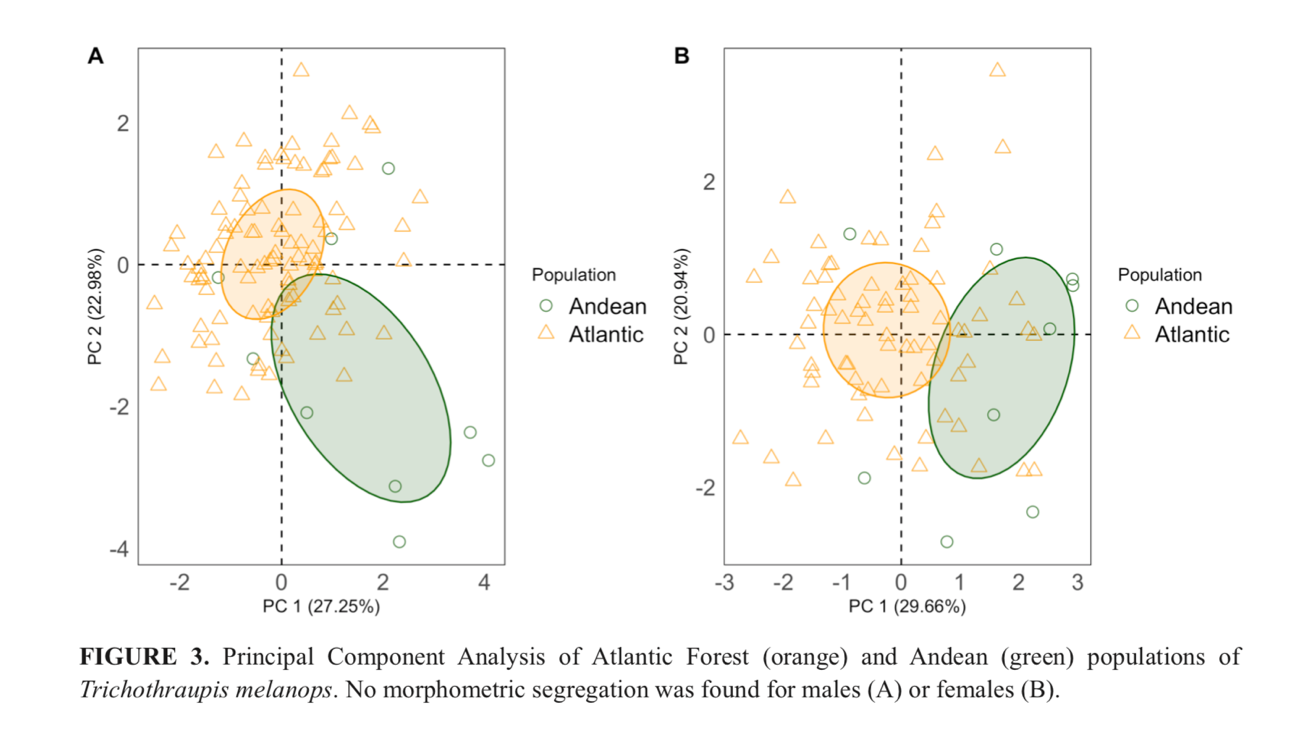
The PCA show wide overlap between the
taxa (i.e., no segregation between taxa when sorted by sex), and univariate
analyses (for which see their Table 1) do not show well-marked differences
(although the authors claim a difference in tarsus length, the effect size for
such a comparison is small). Thus, morphometry show at most subtle differences
between the taxa.
Despite the large number of available recordings, the vocal
analyses included only two recordings of songs of the Andean-foothill
population:
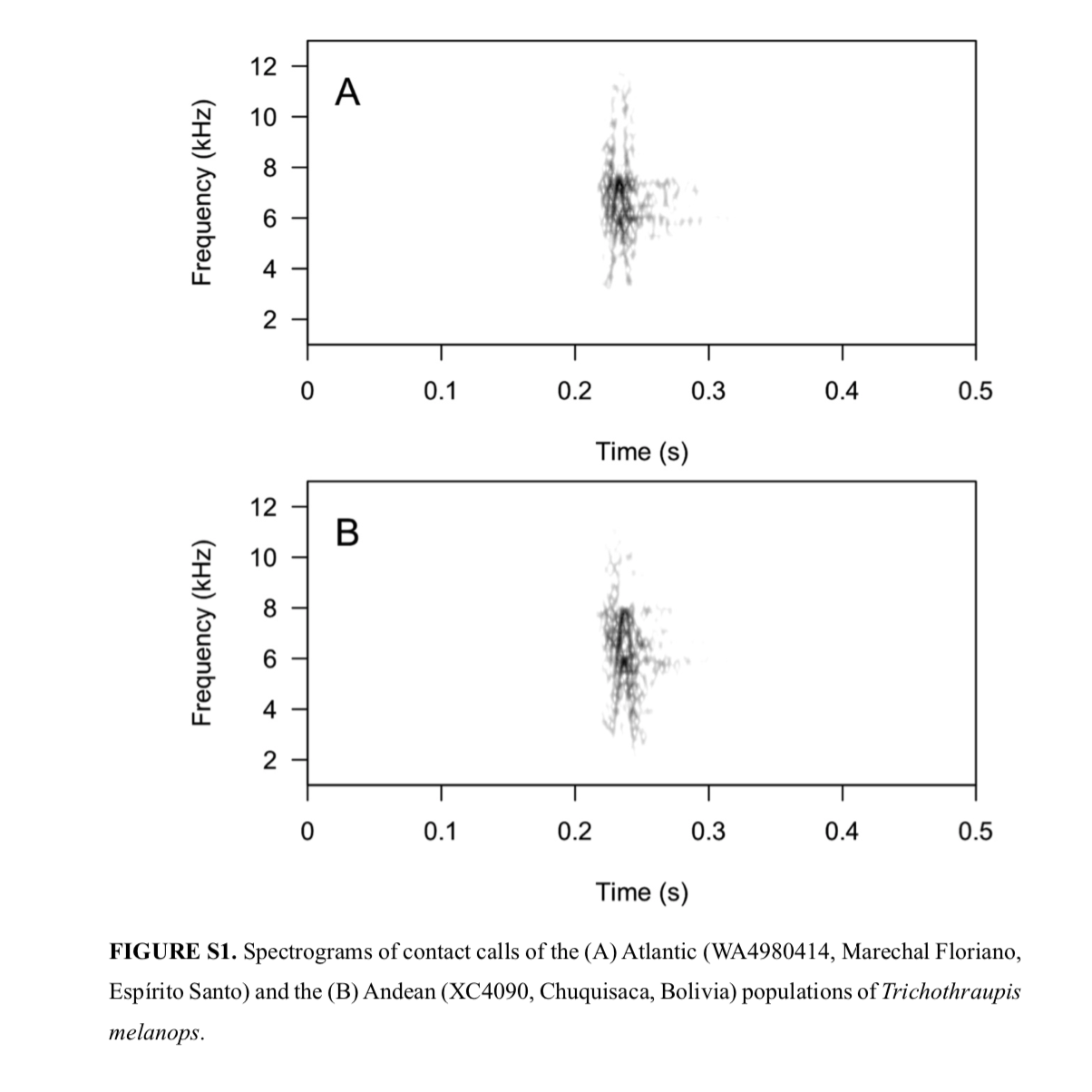
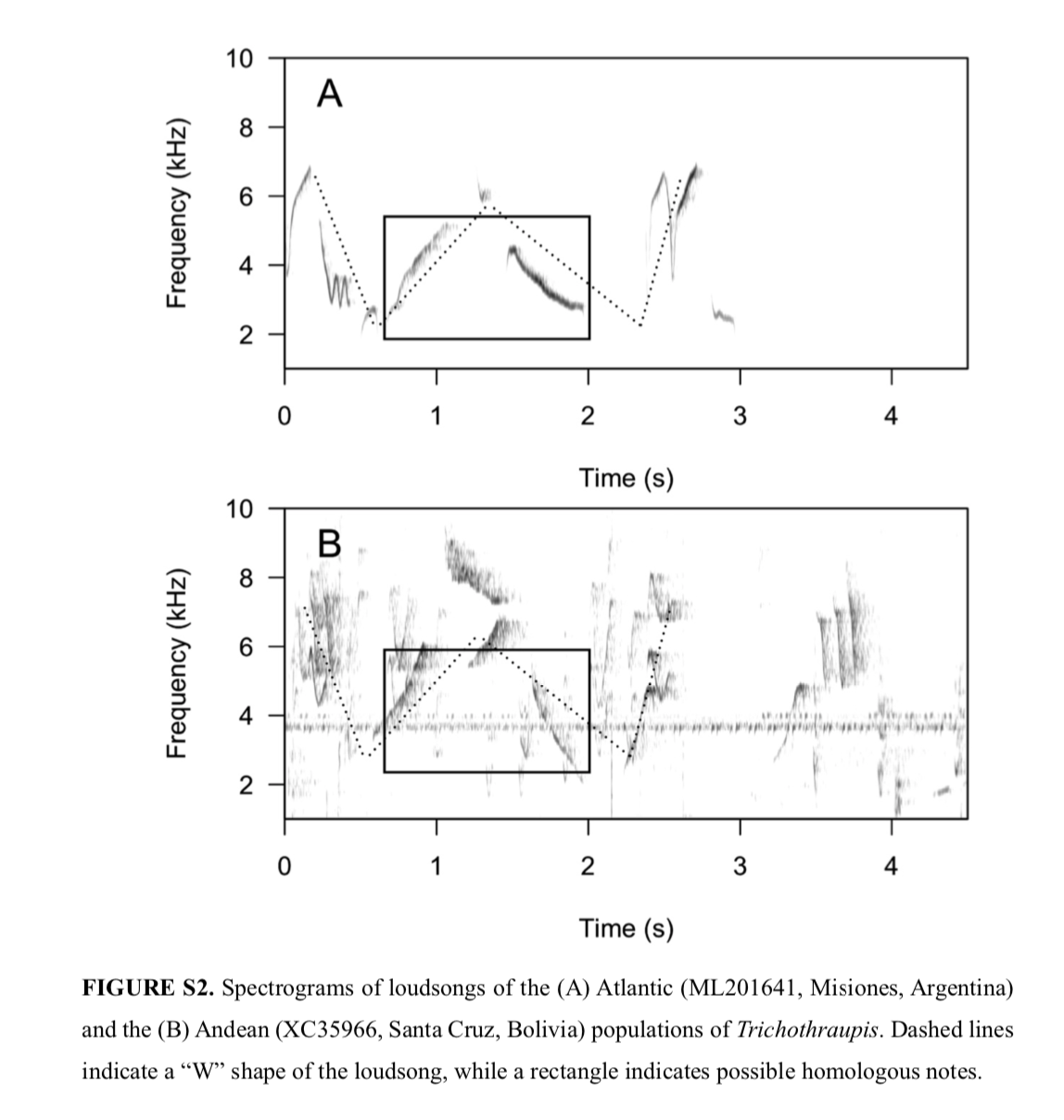
"Vocalizations.
Of the recordings of the
Andean population, only two were loudsongs, and only one consisted of a
reasonable-quality recording to allow for the creation of spectrograms. The
lack of Andean data precluded us from quantitatively analyzing loudsongs.
Qualitative inspections failed to find visual differences between contact calls
(Fig. S1). The Atlantic loudsong is clearly composed of a series of upslurred
and downslurred notes, forming a “W” shape. It seems to share a two-note
homologous pattern with the Andean loudsong, but the number of loudsong
emissions within the recording did not allow for further evaluation (Fig.
S2)."
"Contact calls are visually identical in spectrograms and
sound quite similar for both allopatric populations, but for most passerines,
when sister clades are morphologically and genetically distinct, supposedly
homologous loudsongs also typically differ quantitatively, including for
Neotropical oscine and suboscine species (Isler et al. 1998; Bocalini
& Silveira 2016). Therefore, we also recommend analyzing more loudsongs as
more recordings of T. griseonota are made available. "
The calls of both populations are identical (our own recordings of
both populations from Argentina further reaffirm that), and there was not
enough vocal material to adequately characterize songs (which can be quite
complex in T. melanops, therefore not
an easy task to accomplish).
The genetic data used by Cavarzere et al. (2024) to support their
species-level taxon comes from Trujillo-Arias et al. (2018). Most notably,
their discussion bears on this issue:
"the
divergence between these rainforests in a sample of three birds varied from 1.3
My (Amazona pretrei/A. tucumana, Rocha et al., 2014) to
about 0.72 My and 0.15 My (T. melanops
and Pipraeidea melanonota, this study
and Lavinia, 2016, respectively). However, it should be noted that although the
re-current connections between these biomes could have led to periodic
re-establishment of gene flow between regions after the initial separation, the
historical gene flow between these rainforests has not been high enough to
preclude divergence.
“Finally,
a definitive analysis of the phenotypic variation of T. melanops is
necessary to determine the taxonomic status of its populations. Our results
indicate that the Andean and Atlantic populations of T. melanops are
genetically isolated (i.e., migration between populations M < 1 individuals
per generation), and therefore, its current classification as a monotypic
species might not be adequate to reflect its evolutionary history. "
Of course, these two being allopatric populations, one would not
expect much reciprocal genetic influx. This being said, the genetic
differentiation between the Atlantic Forest and Andean-foothill populations is
very low.
Recommendation: We
recommend a NO vote. The reduced levels of phenotypic and genetic
differentiation coupled with a lack of vocal differences in calls (and unknown,
but apparently trivial levels of vocal differentiation in song; pers. obs.)
indicate to us that griseonota can be
a good, mildly differentiated subspecies of T.
melanops but not a separate species from it.
References
Cavarzere, V., Costa,
T.V.V., Cabanne, G.S., Trujillo-Arias, N., Marcondes, R.S. & L.F. Silveira.
(2024) A new species of tanager (Aves:
Thraupidae) from the Eastern slopes of the Andes. Zootaxa 5468:
541–556.
Hellmayr, C.E. (1936)
Catalogue of birds of the Americas, part IX, Tersinidae, Thraupidae. Field
Museum of Natural History Publications in Zoology 13:1–458.
Trujillo-Arias, N.,
Calderón, L., Santos, F.R., Miyaki, C.Y., Aleixo, A., Witt, C.C., Tubaro, P.L.
& Cabanne, G.S. (2018) Forest corridors between the central Andes and the
southern Atlantic Forest enabled dispersal and peripatric diversification without
niche divergence in a passerine. Molecular Phylogenetics and Evolution
128: 221–232.
Zimmer, J.T. (1947) Studies
of Peruvian birds. No. 51. The genera Chlorothraupis,
Creurgops, Eucometis, Trichothraupis,
Nemosia, Hemithraupis, and Thlypopsis,
with additional notes on Piranga. American Museum Novitates 1345.
Juan I.
Areta and Mark Pearman, July 2024
Vote tracking chart:
https://www.museum.lsu.edu/~Remsen/SACCPropChart968-1043.htm
Comments
from Remsen:
“NO. The authors did a great job of
quantifying the differences between the two populations, especially the newly
discovered diagnostic plumage traits of the two populations. So, the data strongly justify describing the
Andean population as a new taxon … but not as a
species under the BSC but rather as a subspecies. This designation calls attention to their
separate evolutionary history by formal recognition through taxonomy of this
level of biodiversity. What is needed
now, in my view, is a study to show that these two taxa have diverged to the
point of “no return” in terms of divergence in the comparative framework of
Thraupidae divergence through a formal analysis of song differences. Strictly on the basis of genetic differences
and time-since-separation, the data suggest a better fit with subspecies-level
divergence (although I will spare us my usual diatribe on using genetic
divergence as THE arbiter of species vs. subspecies assignment.”
Comments from Stiles: “NO. To me, the
differences in plumage between griseonota and melanops are
sufficient for recognizing the former as a good subspecies, but fall short of
favoring its status as a separate species, and the N = 1 of its loudsong
likewise makes support for the split less convincing. The genetic information
is also borderline and given the records (including specimens?) from Argentina
and Paraguay that narrow appreciably the gap in distributions of the two, any
such specimens should at least be subjected to genetic analyses.”
Comments
from Bonaccorso:
“NO. As Van and Gary pointed out, overall, the data
support a subspecies, not a species. The plumage differences are subtle and the
morphological data overlapping. Also, according to the phylogenetic analysis of
Trujillo-Arias et al. (2018), the genetic differences are minor, and the two
taxa are not reciprocally monophyletic. I don´t mean that reciprocal monophyly
would be necessary, but it would make a stronger case for species status.
Finally, the contact calls and loudsongs don´t seem very different (although I
am not an expert), and not including data from individuals close to the
potential contact zone is problematic.”
Comments
from Robbins:
“For
the reasons Areta and Pearman state in their proposal, I vote "NO"
for treating griseonota as a species.”
Comments from Del-Rio: “NO. Because of low genetic
differentiation and slight morphological differences,
griseonota should be
treated as a subspecies.”
Comments
from Lane:
“A solid NO here. This taxon, while worthy of a name, is hardly distinctive
enough to warrant splitting from T. melanops by BSC standards. This is
why PSC species are a whole different ball of wax from those that most
taxonomists would recognize as distinct.”
Comments
solicited from Kevin Burns: “Cavarzere et al. (2024) is an exciting and important paper
because it reveals previously unreported differences between Andean and
Atlantic Forest populations of Trichothraupis melanops. It’s both
surprising and fascinating that these differences remained underappreciated
until now. In addition, the genetic study of Trujillo-Arias et al (2018)
provides a nice complement. It is a thorough and rigorous study that includes
phylogenies, population genetic analyses, and tests of demographic models.
After reading both of these papers, I’m left feeling this is a borderline case,
but see below.\
“The model
testing of Trujillo-Arias et al (2018) inferred a scenario of peripatric
divergence, with dispersal from the Atlantic Forest to the Andean Forest. This
is also illustrated in their phylogeny, where there is not reciprocal monophyly
between the two sets of populations. Instead, the Andean populations form a
clade embedded within the Atlantic Forest populations. This is what you would
expect in a case of recent peripatric speciation. The timing of the splitting
event is estimated as 0.728 million years ago (mid-Pleistocene). On the
surface, this seems low compared to other avian species splits. However, I went
back to the data from Burns and Racicot (2009), and I found similar levels of
divergence between sister species. In Burns and Racicot, we looked at phylogeny
and biogeography of subfamily Tachyphoninae (Tachyphonus, Ramphocelus,
Lanio, Eucometis, Trichothraupis, and relatives) using cyt
b. Therefore, our dating is based on similar methods and markers as the
Trujillo-Arias et al. study. Several recognized species splits in Burns and
Racicot are of a similar age or even more recent than the Trichothraupis
split. These include Lanio aurantius and L. leucothorax
(0.594 mya), Ramphocelus carbo and R. melanogaster
(0.515 mya), and Ramphocelus dimidiatus and R. nigrogularis
(0.856 mya). Based on these data, the level of divergence seen between the two
proposed species of Trichothraupis cannot be dismissed outright as “too
low”.
“For the
morphological data, there is quite a bit of overlap in the PCA plots of
Cavarzere et al, but the two proposed species do generally fall out in
different portions of the graphs. The authors also describe a significant
difference in tarsus length, but I’m not sure what to make of this. Overall, I
don’t think the morphology adds much to make the case for a species split.
“For the plumage,
the two forms differ in ventral and dorsal color, which is obvious in the
photographs. I wish we had quantitative (spectrophotometric) data comparing
plumage color of all individuals so we could see any potential overlap or lack
of overlap between the two forms. Also, an examination of the color differences
using an avian visual model might be helpful when thinking about differences
and how they might play out in courtship. Nevertheless, I am more convinced by
the differences in the “goggles” – specifically the extent of melanin on the
face mask. There is clear discrete difference in the facemask, with the Andean
birds having black extending onto the auricular. The authors also note that the
crest/crown has black framing in some of the Andean birds.
“Based on the
genetics and plumage differences, these two forms are easily two species under
the phylogenetic species concept.
“So what about
the biological species concept? Well we need to think more about reproductive
isolating mechanisms and try to predict what they would do if they came into
contact. This is the spatial dimension problem that the BSC consistently faces
for geographically isolated populations. We know that vocalizations are very
important in songbirds for reproductive isolation. The authors note that both
species have similar call notes, so that doesn't provide evidence for a split.
For songs, there aren’t enough recordings of the Andean birds for comparison to
Atlantic forest birds. The fact that the Andean birds apparently sing less
(something also mentioned in the Islers’ tanager book) is intriguing. However,
based on available information, vocalizations aren’t helpful in arguing these
are two biological species.
“Since the
genetics shows enough time to evolve reproductive isolation (as seen in related
sister pairs) and the morphology and vocalization data are inconclusive, I
think it comes down to the significance of those plumage differences. For a Yes
vote on biological species, I think you would have to argue that the plumage
difference are important in courtship. For a No vote on biological species, I
think you would have to argue that those differences are inconsequential for
courtship. I don’t think there is much, if anything, known about Trichothraupis
courtship, but personally I haven’t been able to observe them much in the
field. Overall, the differences in plumage seem slight compared to that seen in
other sister pairs in this subfamily. Therefore, if I were asked to vote on
this, I would probably vote No on biological species status for these two
proposed species, although they would qualify under other species definitions.
“Regardless of
species status, I think the Cavarzere et al. (2024) paper is an important one
for understanding diversification and also demonstrates what discoveries await
in museum collections!
Burns, K.J. and Racicot, R.A., 2009. Molecular phylogenetics of a
clade of lowland tanagers: implications for avian participation in the Great
American Interchange. The Auk, 126(3), pp.635-648.
Trujillo-Arias, N., Calderón, L., Santos, F.R., Miyaki, C.Y., Aleixo, A., Witt, C.C., Tubaro, P.L. and Cabanne, G.S., 2018. Forest corridors between the central Andes and the southern Atlantic Forest enabled dispersal and peripatric diversification without niche divergence in a passerine. Molecular Phylogenetics and Evolution, 128, pp.221-232.”