Proposal (1029) to South
American Classification Committee
Revise the
taxonomy of Oceanites species
Current
SACC notes read:
2a. Jaramillo (2003)
suggested that the subspecies exasperatus might be a separate species
from nominate Oceanites oceanicus.
3. A new species, Oceanites pincoyae, “Pincoya Storm-Petrel”, has been described from
Chilean waters (Harrison et al. 2013).
Recognized by del Hoyo and Collar (2014). SACC proposal passed to recognize pincoyae.
3a. Called
"White-vented Storm-Petrel" in Meyer de Schauensee (1970), Hilty and
Brown (1986), Sibley and Monroe (1990), Schulenberg et al. (2007), and
elsewhere. SACC proposal to change English name to
White-vented Storm-Petrel did not pass.
Effect on
SACC: This proposal would split galapagoensis from gracilis,
in addition to exasperatus, and chilensis from oceanicus.
Furthermore, it recognize the taxon Oceanites barrosi sp. nov. as a
valid species. The genus Oceanites, currently with three species, is
expanded to a total of seven species following Norambuena et al. (2024).
Background
& New information: The current classification considers three species within Oceanites:
Oceanites oceanicus, O. gracilis and O. pincoyae. The
species O. oceanicus sensu lato is considered to comprise three
subspecies (Clements et al. 2023): nominate O. o. oceanicus (Kuhl,
1820); O. o. exasperatus Mathews, 1912; and O. o. chilensis
Murphy, 1936. The species O. gracilis sensu lato has two subspecies,
nominate O. g. gracilis (Elliot, 1859) and O. g. galapagoensis
Lowe, 1921, while O. pincoyae is a recently described monotypic species
(Harrison et al. 2013; Remsen et al. 2023).
Previous
studies of the systematics of the genus only included a partial representation
of Oceanites. The first phylogeny of Procellariiformes based on
mitochondrial DNA Cytb (Nunn & Stanley 1998) considered Oceanitidae as a
subfamily of Hydrobatidae despite the evident paraphyly of these clades. This
study only included samples of O. oceanicus (no subspecies specified),
which was recovered as a sister to a clade including Pelagodroma marina
(Latham, 1790), Garrodia nereis (Gould, 1841), Fregetta tropica
(Gould, 1844), and F. grallaria (Vieillot, 1818). Later, Robertson et
al. (2011) incorporated a sequence of O. o. exasperatus into a new
phylogeny of Oceanitidae, which showed a close relationship with an unspecified
taxon O. oceanicus based on Cytb (Nunn & Stanley 1998), and in a
phylogeny based on the 7th intron of b-fibrinogen, O. o. exasperatus was
sister to a clade that includes Fregetta and Pelagodroma (see
Robertson et al. 2011). Robertson et al. (2011) also generated O. g.
gracilis and O. g. galapagoensis sequences, but due to their short
sequences (only 132 bp) these were not included in the phylogeny. Other studies
have only shown the relationships of O. oceanicus (based on Cytb) with
the other species of the family (e.g., Hackett et al. 2008; Cibois et al. 2015;
Prum et al. 2015; Robertson et al. 2016; Reddy et al. 2017) or used O.
gracilis (based on ND1) as an outgroup of a phylogeny looking at the
evolution of Oceanodroma (Sausner et al. 2016).
Phylogenetic analysis:
According to a new phylogenetic hypothesis based on new sequence
data of the mitochondrial gene Cytb and linear morphological measurements of
all species and five subspecies-level taxa in Oceanites (Norambuena et
al. 2024), the genus Oceanites is monophyletic and composed of three
well-supported clades (posterior probability > 0.95): (1) chilensis;
(2) exasperatus; (3) gracilis, pincoyae, and barrosi
sp. nov.; and (4) oceanicus and galapagoensis.
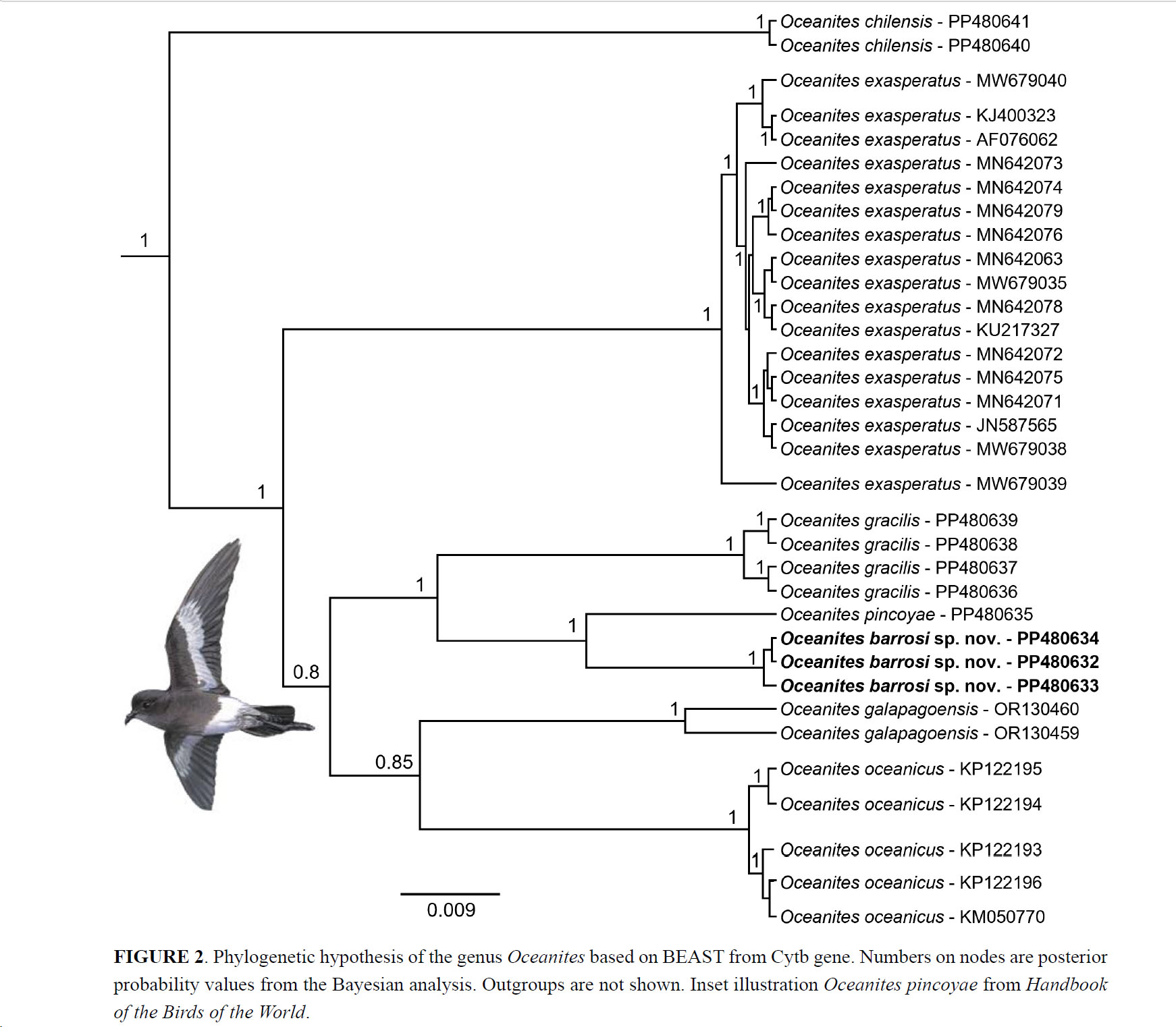
Phylogenetic hypothesis of the genus Oceanites
based on BEAST from Cytb gene. Numbers on nodes are posterior probability
values from the Bayesian analysis. Outgroups are not shown.
This tree also shows that gracilis, galapagoensis, oceanicus,
chilensis, pincoyae, and exasperatus are each
monophyletic. In addition, the populations of Oceanites present in
central Chile (barrosi sp. nov.) were sister to O. pincoyae but
with high divergence (5%). Several of the taxa presently considered polytypic
species are shown to be paraphyletic. The taxon chilensis appears as a
basal clade to the other Oceanites species. Samples from the Andes of
central Chile, form a clade together with pincoyae and are
phylogenetically distant from the samples of chilensis from their main
distribution (close to its Terra Typica - Cape Horn, Magallanes, Chile).
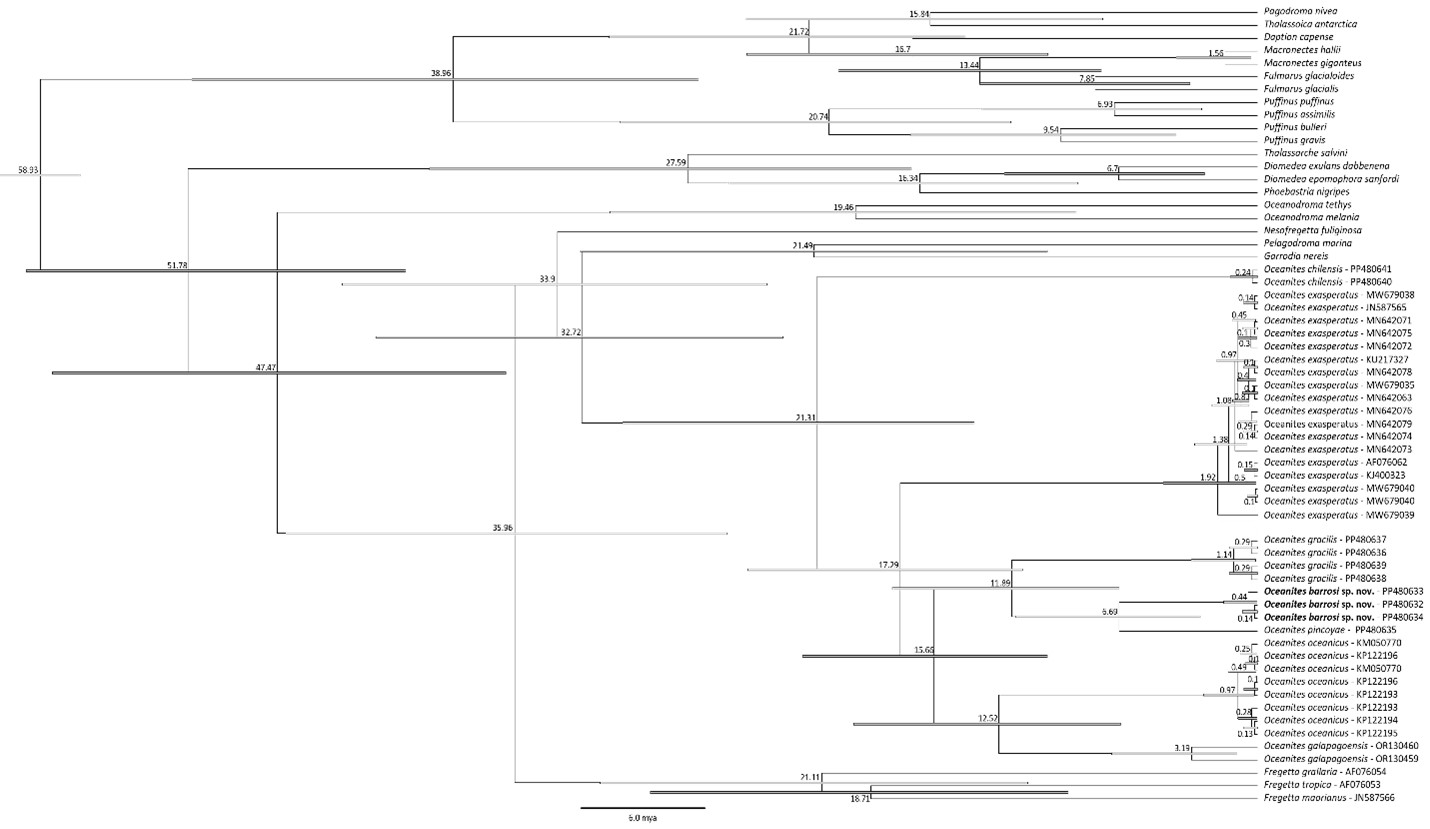
According to a time-calibrated tree (Norambuena et al. 2024), the
split between Oceanites genera and the other genera in Oceanitidae is
estimated at ~32.7 Mya (40.7–22.4 Mya; 95% HPD), and the oldest divergence
within Oceanites (the split between O. chilensis and other Oceanites)
dated to the late Oligocene, around c. 21.3 Mya (29.3–13.3 Mya; 95% HPD). The
most recent split was between O. pincoyae and O. barrosi sp. nov.
dated to the Late Miocene, around c. 6.7 Mya (10.7–2.6 Mya; 95% HPD).
Phylogenetic and morphological data support O.
pincoyae as a distinctive species-level taxon.
Morphological analysis:
For the five measurements: ‘wing length’, ‘tail length’, ‘tarsus
length’, ‘mid-toe claw’, and ‘culmen’ the Bartlett’s test of sphericity and KMO
measures were p<0.001 and >0.78, respectively. PCs 1 and 2 presented the
highest eigenvalues (>0.8) and explained 75.6% of the total variation. PC1
correlated positively with ‘wing length’, ‘tail length’, ‘tarsus length’, and
‘mid-toe claw’ and can be interpreted as a component reflecting overall size;
PC2 correlated positively with ‘culmen’. Scatterplots of PCs showed a gradual
variation between Oceanites species in the PC1 axis, with only marked
differences between gracilis and the oceanicus complex, and overlapping between
pincoyae-chilensis and exasperatus, respectively (Fig. 4). PC2,
or the culmen measures, do not allow for separation of the populations. The LDA
based on PCA results resulted in a 77.3% correct classification of the assigned
species.
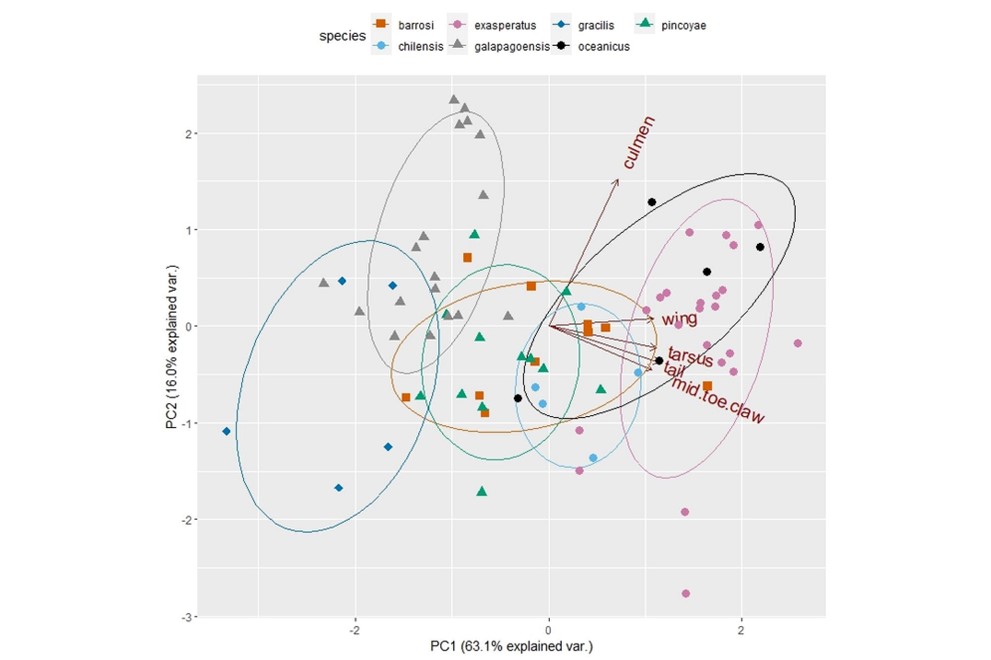
Distribution of average scores between PC1 and PC2 axes of
morphological variation between species/subspecies of Oceanites genera.
Ellipses represent 75% of the variation.
Oceanites barrosi sp. nov.:
Based on the phylogenetic hypothesis and morphological analyses and
following the general lineage species concept, we propose the recognition of
the Oceanites population of central Chile as a new taxon following the
main points presented in Norambuena et al. (2024; see extensive photographic
material therein): (1) Oceanites barrosi wing is, on average, larger
than in O. chilensis but smaller than in O. pincoyae. At the same time, its tail and
tarsus measurements are smaller than in O. chilensis and larger than in O.
pincoyae. Noticeably smaller than O. exasperatus and somewhat
smaller than O. oceanicus mainly in wing and tail length. (2) Restricted
white tips on the belly, never as extensive as in O. galapagoensis, O.
gracilis, or O. pincoyae, but typically not dark-bellied like O.
chilensis, O. oceanites, and O. exasperatus. (3) Bold double
pale line on underwing due to pale tipping on underwing coverts. Underwings are
dark in O. exasperatus and O. oceanicus, and pale tipping not as
bold in O. chilensis. (4) Square-cut tail with conspicuous white,
rectangularly shaped rump patch. (5) In-flight, protruding feet with yellow
webs. (6) Well-differentiated genetically (sister species of pincoyae,
both with 5% of genetic distance). (7) High Andean breeding distribution in
central Andes of Chile above treeline (see Barros 2017).
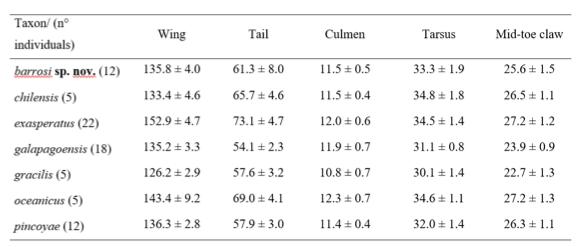
Summary statistics of morphological data
of each taxon within Oceanites. Data are presented as mean ± standard
deviation.
Linear sequencing and English names:
This new phylogenetic hypothesis suggests a new linear sequencing
within the genus Oceanites. Following the criteria of Remsen et al.
(2023); this should be as follows:
Oceanites chilensis (Mathews
1934) – Fuegian Storm-Petrel
Oceanites exasperatus (Mathews
1912) – Antarctic Storm-Petrel
Oceanites gracilis (Elliot
1859) – Graceful Storm-Petrel
Oceanites pincoyae (Harrison
et al. 2013) – Pincoya Storm-Petrel
Oceanites barrosi (Norambuena et al. 2024) – Andean Storm-Petrel – Golondrina de mar
andina (Chilean name)
Oceanites galapagoensis (Lowe
1921) – Lava Storm-Petrel
Oceanites oceanicus (Kuhl
1820) – Subantarctic Storm-Petrel
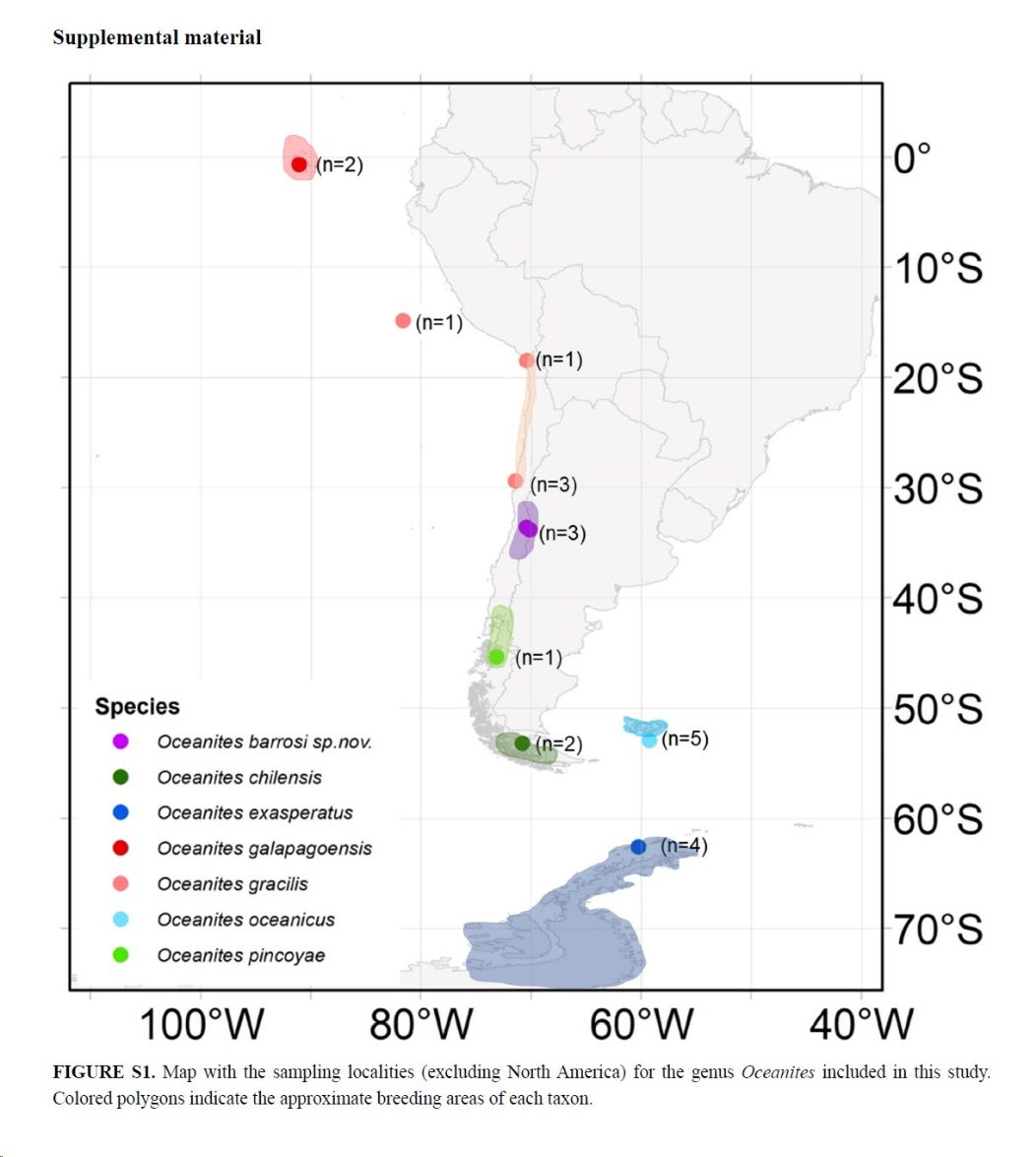
Map of breeding distributions of Oceanites
species in the New World. Note that barrosi and likely pincoyae
are nesting inland in mountain habitats, and similarly gracilis is
inland in the desert. The rest are more marine in their nesting sites.
Discussion:
The elephant in the room here is that all of these taxa vary only
slightly in plumage, with pincoyae the most extreme. They vary in how
much pale they have on the belly, how much pale on the underwing, and how bold
the greater covert bar is on the upperwing. Conservative plumage is the norm in
storm-petrels, and many Procellariformes, and this creates a huge problem for
taxonomists and field observers. We are visually oriented, so we cannot “see”
these species, these seabirds end up being quite a conundrum to us as
taxonomists. But to back track a bit, consider that Leach’s (Hydrobates
leucorhous) and European Storm-Petrel (Hydrobates pelagicus) are
often difficult to separate in the field from Oceanites exasperatus, as
they share nearly the same plumage pattern of brownish body, white rump, and a
pale bar on the upperwing. The similarities are striking, yet these birds are
in different *** families ***!! We need to stress the importance of this point
to put into context the plumage similarities of taxa within Oceanites.
Similarity in plumage is just the norm in storm-petrels.
With regards to the question of biological species, we do have
ample evidence that banded Oceanites return to their colonies and are
extremely philopatric (Bretagnolle 1989), as such geographic structuring of
populations is expected. The birds themselves are known to recognize their sex
and species by vocalizations. However, very little data is available regarding
this point in the literature. Bretagnolle (1989) studied voice of birds from
Adelie Land (exasperatus) and from Kerguelen Island (oceanicus).
He found consistent and statistically significant differences in their voice.
These two taxa differ primarily on size, and breeding latitude, but not in
plumage. One is Antarctic, and one is subantarctic in breeding region. In
addition, he informally notes that other recordings of oceanicus and exasperatus
from additional locations, South Georgia, Crozet Is., and South Sandwich
Islands also show geographic differences. He concludes that “Geographic
variation in vocalizations thus corroborates and parallels the taxonomic
conclusions established from morphological characteristics”. Thus, for two of
our most cryptic species, oceanicus and exasperatus, we have
solid evidence of vocal differences. Bretagnolle and Robisson (1991) created
digitized voices to understand the parameters most important for species
recognition in Wilson’s Storm-Petrel, although they worked in Adelie Land and
Kerguelen, they considered these two populations the same species and as such
worked to understand the underlying parameters in species recognition such as
the duration of syllables vs. silence between syllables. They did not directly
do playback tests to members of each population, which we treat as species.
However, based on the significant differences in voice in parameters that they
consider as isolating mechanisms they note “One may then expect the Kerguelen
Islands birds to be recognized by the Antarctic birds.” Unfortunately, as
multiple of our taxa do not have their nests known yet (galapagoensis, barrosi
and pincoyae), and most others are extremely difficult to visit, voice
is either not known, or has not been analyzed for remaining populations.
Zidat et al. (2017) may have unlocked a key to understanding
reproductive isolation in cryptic Procellariformes. They conducted a study on Calonectris
shearwaters, and not only looked at their morphology, and genes, but the
chemical signal of their uropygial glands. They state, “We also found that
chemical labels remain distinct in sympatry, suggesting their divergence is not
purely due to environmental effects.” The conclusion here is something that
many seabird researchers have postulated for some time, that many
Procellariformes may smell their species. Other examples exist of
Procellariformes recognizing their mates, and homing to their islands based on
smell. We point this out, as it helps to explain why taxa that are well
differentiated genetically, such as our Oceanites, or the Hydrobates
castro complex for example, are so conservative in plumage. It is expected
that the barriers to reproductive isolation will involve smell, and voice.
Ecological differences are worth pointing out. The main one is not
only the latitude of breeding, and adjacent water types, warm, cool, cold or
Antarctic which are ecologically radically different habitats, but also where
they breed. The species oceanicus, exasperatus and chilensis
are on islets, or isolated Antarctic coastline, but definitely strongly marine
in where they breed. The species galapagoensis has no known nest yet,
and they live in the well researched Galapagos archipelago. It is hypothesized
that they are nesting on old lava flows upslope on the geologically newer
islands (Isabela, Fernandina). The species gracilis is breeding mainly
in the desert of Chile and likely Peru, some of them well inland. The new
species barrosi is clearly breeding high up in the central Andes of
Chile based on ample evidence (Barros 2017). Current searches for pincoyae
have clarified that it is likely moving up slope along river valleys, so it is
also inland and likely in the lower southern Andes. This explains the failure
of finding them in islands in Reloncavi. Our evidence, and measurements show
that exasperatus is the expected and thus far only known species that
migrates to the Northern Hemisphere in the non-breeding season. The others are
likely resident or short-distance migrants.
Conclusion:
Based on the phylogenetic hypothesis, and morphological analyses
(Norambuena et al. 2024), we suggest elevating to species status the taxa galapagoensis,
chilensis, and exasperatus, and consider the new taxon barrosi
as a valid species, thus recognizing a total of seven species within the genus Oceanites.
The cryptic nature of these well differentiated species is troubling. However,
the species sort out ecologically based on oceanographic parameters, migratory
behavior, as well as extreme differences in nesting localities. It would be
inconceivable that an island nesting chilensis could magically learn to
migrate to the High Andes like barrosi for example. The little vocal
data available confirms a significant difference between oceanicus and exasperatus
which are two of the most similar species in plumage.
1.
We
recommend a yes vote to accept barrosi as a new species.
2.
We
recommend a yes vote to elevate chilensis, exasperatus and galapagoensis
as species.
3.
We
have recommended a suite of English Names, which perhaps may require a separate
proposal.
References:
Barros, R. (2017) ¿Por qué
aparecen golondrinas de mar en la cordillera de Chile central? La Chiricoca, 22, 4–18.
Bretagnolle, V. (1989). Calls of Wilson’s Storm Petrel: functions,
individual and sexual recognitions, and geographic variation. Behaviour 111:
98–112.
Bretagnolle, V. and Robisson, P. (1991). Species-specific
recognition in birds: an experimental investigation of Wilson’s Storm-Petrel
(Procellariiformes, Hydrobatidae) by means of digitalized signals. Canadian
Journal of Zoology. 69(6): 1669–1673.
Cibois, A., Thibault, J.-C., LeCroy, M., & Bretagnolle, V.
(2015) Molecular analysis of a storm petrel specimen from the Marquesas
Islands, with comments on specimens of Fregetta lineata and F.
guttata. Bulletin of the British Ornithologists’ Club, 135, 240–246.
Clements, J.F., Rasmussen, P.C., Schulenberg, T.S., Iliff, M.J.,
Fredericks, T.A., Gerbracht, J.A., Lepage, D., Spencer, A., Billerman, S.M.,
Sullivan, B.L., & Wood, C.L. (2023) The eBird/Clements Checklist of Birds
of the World: v2023.
Hackett, S.J., Kimball, R.T., Reddy, S., Bowie, R.C.K., Braun,
E.L., Braun, M.J., Chojnowski, J.L., Cox, W.A., Han, K., Harshman, J.,
Huddleston, C.J., et al. (2008) A phylogenomic study of birds reveals their
evolutionary history. Science, 320, 1763–1768.
Harrison, P., Sallaberry, M., Gaskin, C.P., Baird, K.A., Jaramillo,
A., Metz, S.M., Pearman, M., O'Keeffe, M., Dowdall, J., Enright, S., Fahy, K.,
Gilligan, J., & Lillie, G. (2013) A new storm-petrel species from Chile.
Auk, 130(1), 180–191.
Norambuena, H.V., R. Barros, A. Jaramillo, F. Medrano, C. Gaskin,
T. King, K. Baird & C.E. Hernández (2024) Resolving the conflictive
phylogenetic relationships of Oceanites (Oceanitidae: Procellariiformes)
with the description of a new species. Zootaxa
Nunn, G.B., & Stanley, S.E. (1998) Body size effects and rates
of cytochrome b evolution in tube-nosed seabirds. Molecular Biology and
Evolution, 15, 1360–1371.
Prum, R.O., Berv, J.S., Dornburg, A., Field, D.J., Townsend, J.P.,
Lemmon, E.M., & Lemmon, A.R. (2015) A comprehensive phylogeny of birds
(Aves) using targeted next-generation DNA sequencing. Nature, 526, 569–573.
Reddy, S., Kimball, R.T., Pandey, A., Hosner, P.A., Braun, M.J.,
Hackett, S.J., Han, K., Harshman, J., Huddleston, C.J., Kingston, S., Marks,
B.D., et al. (2017) Why do phylogenomic data sets yield conflicting trees? Data
type influences the avian tree of life more than taxon sampling. Systematic
Biology, 66, 857–879.
Remsen Jr., J.V., Areta, J.I., Bonaccorso, E., Claramunt, S.,
Del-Rio, G., Jaramillo, A., Lane, D.F., Robbins, M.B., Stiles, F.G., &
Zimmer, K.J. (2023) A Classification of the Bird Species of South America.
American Ornithological Society.
Robertson, B.C., Stephenson, B.M., & Goldstein, S.J. (2011)
When rediscovery is not enough: taxonomic uncertainty hinders conservation of a
critically endangered bird. Molecular Phylogenetics and Evolution, 61, 949–952.
Robertson, B.C., Stephenson, B.M., Ronconi, R.A., Goldstien, S.J.,
Shepherd, L., Tennyson, A., Carlile, N., & Ryan, P.G. (2016) Phylogenetic
affinities of the Fregetta storm-petrels are not black and white.
Molecular Phylogenetics and Evolution, 97, 170–176.
Sausner, J., Torres-Mura, J.C., Robertson, J., & Hertel, F.
(2016) Ecomorphological differences in foraging and pattering behavior among
storm-petrels in the eastern Pacific Ocean. Auk, 133, 397–414.
Zidat, T., G. Dell’Ariccia,
M. Gabirot, P. Sourrouille, B. Buatois, A. Celerier, F. Bonadonna, P Crochet.
2017. Reproductive isolation maintains distinct genotypes, phenotypes and
chemical signatures in mixed colonies of the two European Calonectris
shearwaters (Procellariiformes: Procellariidae), Zoological Journal of the
Linnean Society, Volume 181, Issue 3, Pages 711–726, https://doi.org/10.1093/zoolinnean/zlx002
Heraldo V. Norambuena, Rodrigo Barros, Fernando Medrano &
Alvaro Jaramillo, July 2024
Note on voting for Remsen: Let’s divide the voting into the three
parts in the Recommendations, i.e.
• Part 1. Treatment of barrosi
as a species
• Part 2. Elevation of three
subspecies to species rank
• Part 3. English names
_________________________________________________________________________________________________________
Comments from Steve Howell: “Comments on Andean Storm Petrel Oceanites
barrosi (Norambuena et al. 2024. Resolving the
conflictive phylogenetic relationships of Oceanites (Oceanitidae:
Procellariiformes) with the description of a new species. Zootaxa
5486(4):451–475)
“First off, I really like the English name for
the proposed new taxon, and clearly there is more to Oceanites taxonomy
than has met the human eye. The authors are to be commended for attempting to
shed more light on this challenging group of cryptic taxa, whose traditional
relationships have undoubtedly been muddled by the human visual preoccupation
of using plumage patterns for taxonomy. For groups such as storm petrels,
morphology (adaptations to oceanic habitats and feeding) plus vocalizations
(for species recognition at night) are surely of more taxonomic import than
plumage patterns. And given that these birds perceive the world through their
nostrils as much as—or more than—in any other manner, it would be great to
develop a way to measure olfactory factors.
“To paraphrase Storrs Olson, however, a more
accurate title for this paper might have been “Towards a less imperfect
understanding of relationships within the genus Oceanites,” and I remain
unconvinced about the new interpretation of ‘species’ limits and whether
another new taxon, let alone ‘species,’ warrants description.
“To some extent, though, this all depends on
which species concept one follows. The authors state that they follow the
“General Lineage Species Concept,” which—based on this paper—I imagine would
result in 50,000+ (or 100,000+?) bird species (= lineages) worldwide. Not that
this is wrong, it’s just a different reality from most current avian
taxonomies; e.g., think of the 35+ “presumptive species” of Gray-breasted Wood
Wren proposed by Cadena et al. (2019; Biological Journal of the Linnean
Society 126:487–506), and so on and on...
“Beyond species concepts and philosophy, I
have a few comments on a paper that apparently was not peer-reviewed (at least,
no reviewers are thanked or listed in the acknowledgements). Some comments no
doubt stem from my own ignorance of molecular methods, and for this I apologize
in advance. In no specific sequence, some thoughts are:
“Sample Size
I don’t know whether using only
mitochondrial DNA from a single gene, and with samples of only 1 or 2
individuals, reveals anything taxonomically meaningful, but my sense from
reading a lot of literature (and NACC and SACC comments) is that gene trees (esp.
using only mtDNA) are not necessarily reliable for inferring species-level
relationships. Thus, it seems a little premature to propose a wholesale
revision of species limits and describe a new species based on only one
mitochondrial gene (which might prove to be biologically and taxonomically
uninformative?), along with molecular samples (for 4 of the 7 populations) that
comprise only 1 to 3 individuals, and with morphological data (for 3 of
the 7 populations) from only 5 individuals. Whether it matters
technically that the type specimen was not one of the molecular samples I have
no idea; the assumption that it is the same taxon is reasonable.
“For meaningful morphometric
analyses, appreciably larger samples should be used; moreover, Table S3 gives
only mean and SD, rather than ranges, thus confusing statistical significance
with biological significance. Consequently, all the fancy stats, principal
component analyses, and figures for morphological differences should be viewed
with caution—and might look quite different with useful sample sizes.
“In this regard, on page 459 the authors disingenuously state “Only three individuals of pincoyae
were assigned to chilensis” in their morphological analysis, but it
appears they had measurements for only 12 pincoyae (and only 5 chilensis!)
which means that 25% were ‘misclassified” = a fairly high proportion. I’m also
unsure what “natural wing length” is? Wing chord or flattened and straightened
wing are the two usual metrics, and they can vary by a few mm between people
measuring them and between live birds and old specimens.
“Taxon Identification
The authors state that “Genetic samples from O.
g. galapagoensis, O. o. oceanicus, and O. o. exasperatus were
obtained from previous studies and GenBank (Table 1).” This assumes specimens
and samples were correctly identified, which is not always true for storm
petrels, cf. fallacious claims made of a close relationship between Black and
Markham’s Storm Petrels based on genetic analysis (Wallace et al. 2017, Molecular
Phyl. & Evol. 107:39–47; cf. Howell & Zufelt 2019:344, Oceanic
Birds of the World) when both samples were from Black Storm Petrels and the
two taxa are, realistically, better placed in different genera! But let’s
assume for now the Oceanites samples used here were correctly
identified. But...
“I see a genuine problem (an interesting one,
though) concerning the (nominate) taxon oceanicus. All (?) authors,
including of this paper, state that oceanicus breeds on subantarctic
[emphasis mine] islands—i.e., places like South Georgia, which has been
designated by some as the type locality of oceanicus. However, the 5
specimens of “oceanicus” used in this paper are from the Falklands,
which are not true subantarctic islands and are biogeographically more
similar to Cape Horn and the Fuegian zone of southern Chile, cf. author’s
Figure S1. Based on morphology, plumage, and biogeography, other authors
(Howell & Zufelt 2019, op. cit., Howell in press, Marine Ornithology)
have provisionally suggested Falkland-breeding Oceanites may even be
referrable to a broadly defined “chilensis.”
“This is not to say, however, that Oceanites
breeding in the Falklands aren’t oceanicus that travel to forage in cold
subantarctic waters—oceanic birds are adapted to marine habitats and use the
nearest suitable land for nesting—but using birds from South Georgia or another
truly subantarctic location would have been better—indeed, such birds might
prove different again from Falkland birds, who knows? For example, some
Falkland seabirds are endemic taxa or potentially separate species, distinct
from populations/taxa at both Cape Horn and South Georgia/true subantarctic
islands, e.g., “Common Diving Petrels.” Moreover, oceanicus breeding on
different subantarctic islands (e.g., South Georgia, Crozet, Kerguelen) vary in
measurements—in some cases as much as taxa recognized in the present study—and
with appreciably larger samples (Marchant & Higgins 1990; HANZAB
vol. 1).
“Thus, I argue that true (nominate) oceanicus
was not examined in this paper, and that the taxonomic ID of Falkland breeders
remains to be elucidated. Also, samples from various true subantarctic islands
should be included in any comprehensive study of the complex.
“Biogeography
Nice things the paper reveals include the
‘closeness’ of mainland-breeding “pincoyae” and “barrosi” and gracilis,
which makes biogeographic sense, although (pending larger samples and more
thorough analysis) it may be that “pincoyae” is simply a southern
subspecies/variety of “barrosi,” one in which a ‘white morph’ is
frequent (as with dark morphs being frequent in southern-breeding Leach’s Storm
Petrels Hydrobates leucorhous). If that proves to be the case, the wide
plumage variation in presumed “pincoyae” (inexplicably overlooked by
Harrison et al. 2013, cf. Howell & Schmitt 2016, Dutch Birding
38(6):384–388) would be explained (cf. Flood et al. 2024, Marine Ornithology
52:165–171; Howell in press, op. cit.); and then barrosi would
become a junior synonym of pincoyae... The English name Andean Storm
Petrel for both taxa combined, however, would still be appropriate.
“Thus, we would have chilensis adapted
to cold Fuegian waters, “pincoyae/barrosi” adapted to cooler southern
Humboldt Current waters, and gracilis adapted to warmer northern
Humboldt Current waters.
“I also find intriguing the suggestion of galapagoensis
(n = 2) and (nominate) “oceanicus” (n = 5) being sister taxa. However,
a) true oceanicus was not necessarily sampled, and b) this purported
close relationship may prove spurious when more genes are sampled.
“On p. 463, the authors state: “The results
support the validity of the taxon O. (oceanicus) exasperatus, and its
plumage similarity with O. (oceanicus) oceanicus sustains the hypothesis
that exasperatus is a sibling or cryptic species...” but sibling to
what?
“Additionally on p. 463, the authors state
“Given our phylogeny and that a sample from North America falls within the exasperatus
clade, it is likely that only exasperatus migrates to the northern
hemisphere while all other forms are resident in the southern hemisphere...” Is
there any evidence that true (subantarctic) oceanicus (e.g., from South
Georgia) remains in the southern hemisphere? Perhaps it does, but a single
sample from North America is tenuous support for such a sweeping statement.
Either way, this would be an interesting thing to establish, along with how the
wing molt strategy of true oceanicus might differ from exasperatus
if the latter is the only transequatorial migrant Oceanites (cf. Howell in
press, op. cit.).
“Summary
Until a more thorough analysis of the Oceanites
complex is conducted, with appropriate samples and potentially more informative
molecular analysis (and ideally song samples, and playback—dream on!), this
study is tantalizing but inconclusive.
“To my mind, recognition of “barrosi”
and “pincoyae” as (biological) species still awaits critical and
unbiased study; as noted by Howell & Schmitt (2016, op. cit.), most of the “unique” specific features
attributed to “pincoyae” (behavior, foraging ecology, molt timing) are
either spurious or uncertain; even plumage characters of presumed pincoyae
may overlap completely with presumed chilensis (now = presumed “barrosi”?)
(Flood et al. 2024, op. cit.).
“To those with historical knowledge of
storm-petrel taxonomy and nomenclature, the saga of naming different
populations of Oceanites in central and southern Chile, based on limited
samples, brings to mind the naming of various different taxa of Leach’s Storm
Petrels in western North America in the last century.
“Indeed, I wonder whether a study using the exact same
methods and sample sizes as used by Norambuena et al. for Oceanites,
but conducted on different populations of Pacific coast Leach’s Storm Petrels
from Alaska to Mexico (not including Townsend’s and Ainley’s) might not result
in one or more new ‘species’ of Leach’s being recognized? Again, who’s to say
what’s ‘right’ or ‘wrong,’ although multiple species of Leach’s doesn’t accord
with what we know of the biology (e.g., songs from Alaska to Mexico sound similar), despite a wide latitudinal range.
Conversely, song playback tests for Band-rumped Storm Petrels reveal good
morphological, ecological, and biological species that myopic molecular
analyses failed to reveal; cf. Howell 2021; North American Birds 72(1):16–25).
“In
conclusion, real-time biology and molecular sampling do not always align, and
there is still much to learn about storm petrels!”
Comments from Areta:
“A) YES to O. barrosi. Although
the type specimen from Río Blanco was not sequenced, the three birds sequenced
came from close enough to the type locality (e.g., Río Colorado is some 30km
from Río Blanco) as to make it sound very reasonable that the authors sampled
the same breeding population. As far as we know, there are not two different
taxa breeding in the same or nearby burrows, which seems to be the main risk of
not having sequenced the type. Thus, it is a judgment call that I am willing to
accept at this point, while hoping for this holotype to be sequenced sooner
than later. The 5% divergence reported in Cyt-b is reasonably large in relation
to intra-taxon variation (see for example the rather homogeneous genetics of
the Antarctic exasperatus). In terms
of divergence, barrosi
sits on the lower end of differentiation among the species
recognised by Norambuena et al. (2024), and by this yardstick (and in the
absence of vocal data) there is room to discuss whether
barrosi would
better be considered a subspecies of pincoyae or a
separate species. Dealing with cryptic species that have very similar plumages
and morphology of course will cause headaches when trying to identify the taxa
in the field. I wonder whether some of the questionings of Howell & Schmitt
(2016; Dutch Birding, discussed here https://www.museum.lsu.edu/~Remsen/SACCprop721.htm) regarding
the unexplained variation in individuals of
Oceanites "oceanicus"
(and their skepticism towards
pincoyae as a
separate species) could be explained by the existence of
barrosi. There are some unknowns here that make the case less clearcut
than the 3-way split discussed in B, however, the data collectively tips the
scale towards recognition of barrosi
as a species, and so I vote to accept it. It also remarkable that
there is at least one specimen from Argentina (Las Cuevas, Mendoza) that needs
proper assessment (Mark Pearman is working on this), but which may prove to
belong to barrosi
(see map of Oceanites
oceanicus in Pearman and Areta 2020
where such a specimen has been indicated by a circle). Barros (2017) discussed and showed
photographs of this specimen (http://www.lachiricoca.cl/wp-content/uploads/2018/03/CHIRICOCA-22-Golondrinas.pdf)".
“B) YES to the recognition of
exasperatus, chilensis, and
galapagoensis as separate
species. The three have long been recognised as distinct taxa with drastically
different breeding areas, and the genetic evidence in Norambuena et al. 2024
indicates deep splits. Although no doubt future genomic analyses will provide
further clarifications, the mitochondrial data is enough for me to push these
taxa to species level.
“C) Hmmm. Though I am not a voter, I think I prefer to call
barrosi something
like "Aconcagua Storm-Petrel". The Andes harbour several species of
breeding storm-petrels, so it does not seem to be particularly informative to
call it Andean Storm-Petrel. As for gracilis, the
scientific name seems to refer to it being
gracile, which is not the same as graceful
(also: is there a need to change the name Elliot´s?) . Why "Lava"
instead of the more prosaic "Galapagos"? Yes, there is a Lava Gull
and a Lava Heron, but... “
Comments solicited from Hadoram Shirihai: “I saw
your [Steve Howell] comments now on the SACC, with which I agree 100% on every
point!
“I can add that in relation to point 6 below, between 2013 and
2019 I did quite an extensive survey, year round, of much of pelagic waters and
islands off Chile, the Humboldt Current and beyond. Some of my works you know.
The unknown large birds that I am reporting in point 6, between Nov and Mar,
are from off c. Chile, including pelagic waters off Valparaiso. The vast
majority are of such examples. Many of them have variable degrees of pale
ventral area - BUT they are large and by far so, from the measurements given
for the new species barrosi - see measurements in point 6! I include for
you screenshots from the pages of one of the unpublished reports of the work.
So, based on the data they give in the article for barrosi sp. nov. (the
Andean Storm-Petrel), such birds as the latter from the Andean Mts east of
Santiago, same season, by and large do NOT exist in the ocean
below. They do NOT!!! Or their measurements in the paper are completely
wrong.
“Amazingly, we think the same also about the Antarctic and
Sub-Antarctic; this is what I wrote to Alvaro on FB yesterday:
"one more comment, for
birders going to or already have visited South Georgia and will use this paper
to try to guess what kind of 'Wilson's Storm-Petrel' they are seeing: this new
paper suggests that 'oceanicus' is the form of the subantarctic islands
including South Georgia, Falklands, etc. But my data are clearly showing that South
Georgia and the Falklands are very different in biometrics, and with the former
much closer to the Antarctic birds. So, if one is going on a voyage to South
Georgia, he or she cannot just automatically 'tick' it as 'Subantarctic
Storm-Petrel', as this paper suggests, as it is NOT, or not sure yet! Secondly,
the type locality of the oceanicus is unclear, so this needs to be sorted
out, too."
“Last point, based on taxonomic thinking we advocate for the
gadflies, and based on what we know so far about Oceanites spp., and if
I take your idea that maybe plumage variation is the least important, but not
size differences, I would only describe these four taxa as three ecologically distinctive
conservation units, as: gracilis (1), pincoyae-barrosi (2), and galapagoensis
(3). But I will have a better view once Vincent and I can process much of the
data collected over the years.”
“The main obstacles with
the recognition of the new Oceanites
barrosi sp. nov. Andean Storm-Petrel (Norambuena et al 2024, Zootaxa 5486
(4)) are:
“1. The selected type specimen LACM 25182),
and other similar pale-bellied birds that I handled inland, near and east of
Santiago, have both measurements and plumage features strongly approaching gracilis
(Elliot’s Storm-Petrel). The type is by far more white-bellied, like gracilis
and pincoyae, while the other birds shown in photos from traps in the
same area are much darker, even without any pale ventral area.
“2. The paper lacks genetic
check/comparison of the type specimen LACM 25182, and the birds sampled for barrosi
in the tree in Figure 2 not shown in images too. I see the danger that both
sources are of different taxa?!
“3. So, I am left with no other choice to
question the description of barrosi, and to ask how I should refer to
the birds inland (same area of the type locality), near and east of Santiago
with wings 126-128 mm and plumage like gracilis too?
“4. The work selected two paratypes, the
ones from the Santiago museum, but they overlooked (or avoided) that in the
same cabinet in the museum there are two specimens from the same area inland that
are small and very much gracilis-like in plumage.
“5. With Fig S2 (in page 471, Calibrated
phylogeny of Oceanites), the work compared the dated splits with other
tubenose pairs. Here for comparison, they calculated 1.56 MYA between the
Northern Giant Petrel M. halli and Southern Giant Petrel M. giganteus.
However, now several studies have found that the divergence of these have
occurred only approximately 0.2 MYA. That is about 90% discrepancy using the
same marker, Cyt-b. I am unsure how they got the 1.56 MYA for the
latter? For the split of pincoyae versus barrosi, they give 6.69
MYA, but if a comparative 90% error might apply, the true divergence might be
down to about 0.5 MYA, or less. And yet, this is just by Cyt-b, but if
you used nuclear markers, it will probably will not be more than a few thousand
years, just as with many other tubenose pairs. So, I suspect that the work have
by over 90% miscalculated (overblown) the dated splits, which have mislead them
altogether!
“6. The paper gives barrosi
(in TABLE S3, form birds trapped in Andean mountains east of Santiago) as
having an average wing 135.8 mm and tail 61.3 mm. Such birds, however, I failed
to find along the Humboldt Current, including pelagic waters off Valparaiso,
from 19 November to 16 March, i.e. in the same waters, same dates, virtually
only large-sized Oceanites [oceanicus] complex birds with average
wing 148.7 mm and tail 65.1 mm, i.e. by far larger than the new barrosi
sp. Nov. (I have samples of such birds, and I am unsure of their origin,
pending analysis).
“7. My suspicion is that because they
miscalculated the dated splits, they concluded that pincoyae and barrosi
are different species, and also both from gracilis, but in fact all
three represent variation of same thing, gracilis! Their tree (Figure 2)
is confirming that, but it just overblown the divergence between them!”
Response from Heraldo
V. Norambuena: “It is interesting to see the interest that our work
with Oceanites has generated, and we hope that it will be the starting
point for a more in-depth analysis of the evolution and speciation processes of
this fascinating group. Some points to clarify/answer:
1)
As
clearly indicated in our article (Norambuena et al. 2024) and the proposal, we
define species based on our phylogenetic hypothesis and morphological analysis,
not just on morphology. For those more comfortable with the concept of
morpho-species, it is expected that our results will not please them. If we
were to base ourselves on morpho-species, we would have 8,000-9,000? described
species (as in the period before Sibley & Ahlquist 1990), and multiple
proposals submitted to the SACC would not have been validated. We agree that
vocalizations are essential for species delimitation. Indeed, we have described
some vocalizations for Oceanites gracilis (Barros et al. 2020), and we
have acoustic monitoring with ARUs in progress with Markham’s and Ringed
Storm-petrels. Still, we must be realistic because it is a significant
challenge for seabirds to generate this data. First, we need to find the
breeding sites of barrosi and pincoyae, we are close, just as we
have already done with several Atacama Desert storm-petrels (e.g. Schmitt et
al. 2015, Barros et al. 2019, 2020, Medrano et al. 2019, Norambuena et al.
2021). Our species delimitation is not based on Figure S2 and its divergence
times but on Figure 2, which shows a topology and branch lengths representing
the cyt-b-based genetic divergence for each clade. Mr. Shirihai is, therefore,
misinterpreting the phylogenetic trees. The monophyly of gracilis
concerning pincoyae and barrosi is quite evident in Fig. 2, so
the criterion of considering this large clade as gracilis alone does not
make sense with phylogenetic/evolutionary species concepts (see de Queiroz
1998, 1999, 2007). I would agree with this point if the phylogeny showed a
clade with no taxonomic or geographic correspondence and slight genetic
divergence (as occurs with the clade of exasperatus in our Fig. 2), but
that is not the case. It should be noted that a multispecies approach such as
ours provides greater clarity of the process of cladogenesis or speciation than
one based on population genetics of a single taxon (as Mr. Howell points out
concerning Leach’s Storm Petrels; there is an analysis scale error!). We
decided to calibrate the phylogeny to explain the biogeographic process behind
the speciation of the group to understand better certain relationships such as galapagoensis/gracilis,
which, according to our analysis in BioGeoBEARS is due to a jump-dispersal
event, due to a long-distance colonization event in biogeographic contexts
quite different from what we see today (marine introgressions before the Andean
uplift were quite frequent in Chile and Argentina, you can see a review here
Hoorn et al. 2022 https://doi.org/10.1093/botlinnean/boab098). As Howell
points out, it is essential not to forget the biogeography behind these
processes, not only the current but also the historical ones.
2)
Measurements:
Regarding this criticism and the exaggerated assertion that there is no bird of
such size in the ocean, we can specify that our measurements for barrosi
were mainly taken from birds captured with mist nets in the mountain range near
their breeding area and skins from land near those areas, in Río Blanco (6; 2
of them are part of the type series), Río Colorado (2) and El Morado (1). We
considered the measurements of three specimens from the Museo Nacional de
Historia Natural de Santiago: MNHNCL3606 from Cerro Manquehue, Santiago
(paratype, female, Nov-1966), MNHNCL3500 from Aconcagua (paratype, male,
15-03-1961) and MNHNCL1957 from Santiago (male, Feb-1944). The specimen
MNHNCL3606 measures 143mm in the wing and 58mm in the tail; therefore, O.
barrosi individuals can reach the measurements that Mr. Shirihai indicates
(we measured ‘natural wing length’). Mr. Shirihai argues that there are gracilis-type
specimens in the collection but does not indicate the location or number of the
specimens. It should only be noted that there are indeed three specimens
labeled as gracilis, two from Mejillones, Antofagasta, 1,400 km away
from Santiago (MNHNCL4673 without sex from 19-12-1982 and MNHNCL4674 without
sex from 20-12-1982), a third MNHNCL4722 with locality Pudahuel, Chile (without
sex, 17-05-1982). Our measurements for that specimen (MNHNCL4722) are 126 mm
wing and 55 mm tail, which coincides with what Mr. Shirihai points out, with
the difference that the amount of white on the belly does not correspond to gracilis;
on the contrary it shows the pattern that we describe for barrosi (we
attach a photo of the specimen and a comparison with gracilis captured
in northern Chile). For O. gracilis, we included in our analysis three
birds captured at their breeding sites on Isla Chungungo and Pampa del Indio
Muerto; information on that species is published in Barros et al. 2020
(https://doi.org/10.5253/arde.v108i2.a7). Of all the skins reviewed, we
discarded about 50 specimens from our analysis because they did not have a
precise location on the labels (many from AMHN), or because they were caught at
sea, for example, USNM skins collected in “Pacific Ocean off Peru”. Therefore,
our sample at this point is low because we were picky in selecting the skins to
consider, and the data are mainly adjusted to data from birds captured near or
at the breeding sites. Many data reported by Harrison et al. (2013) were not
included because they did not consider Mid-toe+claw a relevant measure. At this
point, I would like to clarify that measurements and captures of birds at sea
will most likely be misleading because, as we pointed out on page 463 of our
paper, the distribution of each taxon at sea must be assessed with the
appropriate technology (e.g. geolocators). At certain times of the year chilensis/pincoyae/barrosi/exasperatus?
could coincide in some regions of the Pacific (or Humboldt current) (I know it
is a problem, but it is a great challenge to solve with science.) Any
morphological data discussed should precisely indicate the number of specimens,
location, and date. At the ROC, we collaborate with institutions that are
rehabilitating birds affected by light pollution in Santiago and northern Chile
(birds captured on land require capture permits), so in future works, we will
be able to have more information for several species (the data from our
publication are available upon request).
3)
Holotype:
It is an excellent point to sequence the holotype. I recognize it was a mistake
not to have done so, but that does not invalidate the pattern evident in the
phylogenetic analysis. We will consider this observation and ask for samples
from the LACM specimens and the MNHNCL to sequence the entire type series. The
description of the species requires us to define a morphological standard, but
as in any population, the distribution of the traits will (or should) have a
normal distribution, with an average of specimens adjusting to that standard
described (Diagnosis) and with specimens that deviate from that average, with a
greater or lesser amount of white, and that also applies to sizes. The proposal
discusses that point, but I can add that O. barrosi does not have a
white patch on its belly, as O. pincoyae and O. gracilis do. Oceanites
barrosi have white feathers that may stand out between the belly feathers;
this white may be more evident in museum skins because the taxidermy process
disarranges these feathers, the "gracilis" close to the type
locality including those pointed out by Mr. Shirihai are indeed barrosi.
4)
Calibrated
phylogeny: On this point, Mr. Shirihai does not cite a source. The only work
that explicitly shows the divergence between Macronectes halli vs. M.
antarcticus is by Techow et al. (2010), who points out a divergence of 0.5 MYA,
not 0.2 MYA, as Mr. Shirihai points out. Therefore, the discrepancy he
indicates is miscalculated. In our work, we are critical of the shortcomings of
these calibrations, and we suggest that this is a point to be improved with a
multilocus or genomic coverage (last lines, first paragraph, page 464).
Regarding the result, in addition to the uncited example indicated by Mr.
Shirihai, multiple examples with Procellariiformes indicates quite old ages for
several clades of this group (e.g., Gangloff et al. 2012, Wallace et al. 2017,
Estandía et al. 2021 pre-print). Some studies have shown that cyt-b-based
calibrations often estimate older dates than multilocus or genomic ones (we
have experience with Neotropical Pipits, see van Els et al. 2019, Norambuena et
al. 2018, 2021). Still, those differences do not give variations from 6 MYA to
0 MYA (that is why the tree must be based on Bayesian analysis because it is
the most accurate way to have reliable and informative branch lengths); if that
were the case, the topology of the phylogeny based on cyt-b or another
mitochondrial gene would be closer to a polytomy with very short branch
lengths, and that is not the case with our phylogeny with well-resolved clades.
SACC members have enough experience to draw their conclusions.
5)
Finally,
for the Atlantic taxa and populations, the lack of clarity of the 'terra
typica' of oceanicus is a problem with no solution. I agree that it is
essential to understand well what happens with Malvinas/Falklands – South
Georgia – Isla Los Estados, but you will realize that considering our results,
we had to propose something and biologically Antarctic vs. Sub-Antarctic makes
sense, Bourne (1964) and Murphy & Beck (1918) had already pointed this out
in some way, and we cited both in the discussion of our article. However, I
agree that it is necessary to obtain samples from South Georgia and other
sub-Antarctic islands (I hope that colleagues working on that side will achieve
it soon!). But again, oceanicus (or what should be oceanicus) and
exasperatus are phylogenetically diagnosable.”

Comments
from Manuel Marín:
“Morphometric data. The
authors provided morphometric data on 79 individuals. However, 37 of these were from live,
mist-netted birds, whereas the remainder were from museum specimens. Comparing measurements from live birds to
specimens is problematic because of known shrinkage of museum specimens. Further, within each of those two categories,
no indication is given in terms of who measured the specimens, other than “all
the information in the database is unpublished and was measured by H.V.N. and
R.B.”. If each sample was measured
by the same individual, then that should have been mentioned in the
Methodology; if more than one person measured specimens within each category,
then that also should have been mentioned because individual differences in the
way specimens are measured is a known source of error that can be important
when measurement differences are close.
This may not have affected the results at all, but as is, we have no way
of assessing this potential problem.
Because no museums are in the Acknowledgments for assistance in
providing measurements or for access to the collections, the measurements must
have been done during visits to those collections by H.V.N. or R.B.
themselves. The Zootaxa review
process let the authors down on not clarifying these details (and not requiring
the customary courtesy of acknowledgements to curators of the collections used).
“Type specimen: I know the type specimen well and also the other older specimens
mentioned in the paper. In 2000, I
identified the type as O. gracilis (still on the label in my writing)
for a paper on seabird vagrancy. The
identification was on the base of size and the white feathers on the belly.
Type specimen: LACMNH 25182: (my measurements) Wing 136; tail 59; tarsus 30.2;
culmen 10.8.
“At the time I compared it to 11 specimens from Cape Horn for O. o.
chilensis. However, I failed myself to notice the Oceanites paper by
Murphy (1918; Bull AMNH 37:117-146), in which he discussed age classes and
plumage: A) young birds have white feathers on the lower belly but through
aging they become dark – pretty much like Cypseloidine swifts. B) and there is a size difference – the young
birds are smaller than older ones.
Norambuena et al. labeled the type specimen as adult. However, in my
notes I wrote that it has DOWNY feathers on the lower body and flanks—and my
goodness if you look carefully at the picture, they are there, and they can be
seen as you zoom in. Indeed, the type is a young of the season, in my opinion.

“Plumage variation: Although Murphy (1918) is cited (as Murphy & Beck 1918) with
respect to plumage variation, the problems that Murphy discussed were not dealt
with directly in the paper’s analysis of
plumage and morphology. Here are the
relevant sections from Murphy:
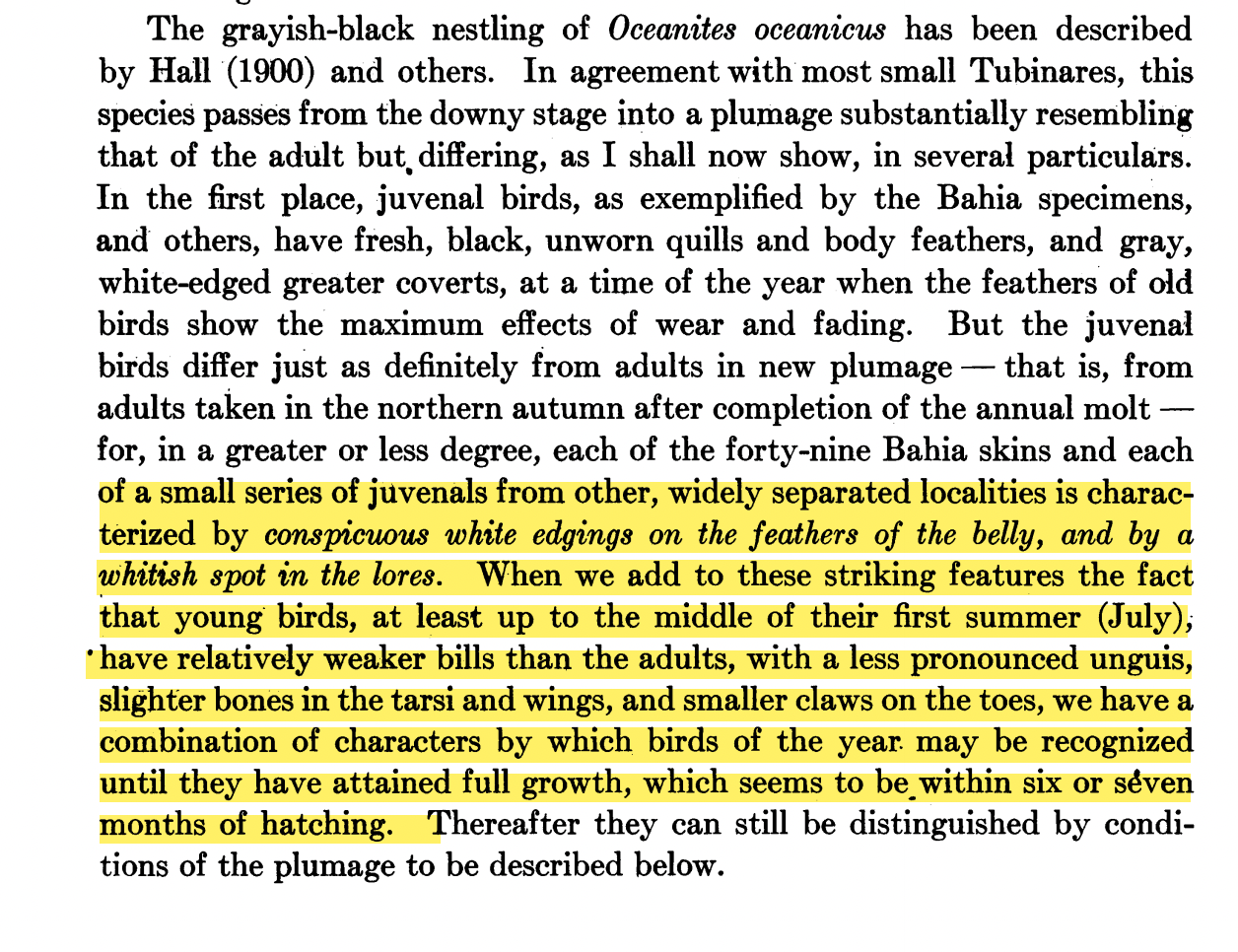


“In the same paper on page 126,
Murphy referred to young individuals that are smaller and have weak unguis. ¨These features, added to those of
its juvenal plumage (which seems the case here) give the skin a sufficiently
different facies from the well known adult form of Oceanites o. oceanicus
to serve an ornithologist who was unacquainted with the characters of a juvenal
bird as the type of a new race. ¨ In this
respect I agree with Howell & Schmitt (2016) that is premature to refer to O.
pincoyae as a full species, but equally applicable to O. barrosi. I
have been watch closely for the ¨Pincoya¨ type, and I have photos of classic ¨Pincoya¨
types (e.g., white underwings and white from lower chest to belly) at different
locations as far north as central Chile area (which would mean a considerable
range extension) where ¨barrosi¨ is supposed to be. The degree of variation as mentioned by
Howell and Schmitt (2016) is quite large and needs to be addressed before
making taxonomic conclusions. An alternative hypothesis is that needs to be
addressed directly is that all might turn out to be different age classes of chilensis.
Keep in mind that seabirds are not like most landbirds and can take many years
before acquiring true adult plumage and before first breeding and might stay
for long time at sea before returning for first breeding.
“Misc.: The idea of nesting in the Andes is highly plausible as they
are not that far from the coast (110 -140 km), not much for a seabird, and the
high Andes emulate the same climatological environment as nesting far
south. However, this still needs to be
proven; ss far as I know there is no direct evidence whatsoever, as many (if
not all) of the individual birds captured inland relate to some weather
anomaly, e.g. see Marín 2002 (‘The occurrence of vagrant seabirds inland in
Chile’. Cotinga 17: 62-65).”
Comments from David Ainley (voting
for Bonaccorso): “#1 --- NO, for #2 --- NO, and for #3, I suggest that all the
renaming hold off until questions 1 and 2 are resolved. On the other hand, I’m
not a taxonomist, though I stuck my toe into the pool a couple of times in the
past. But I did rely on others to save me from drowning, like Storrs Olson.
“Storm-petrels distributed across
an extensive, latitudinal space, e.g. Leach’s, show much plumage and size
variation, in somewhat of a cline along NA west coast. However, this has not
resulted in a parsing into a series of ‘species.’ Pretty much color morphs. The
Atlantic and western Pacific don’t show the same extreme of ocean climate
variation as either the eastern North Pacific or the eastern South Pacific,
somewhat the product of eastern boundary currents. Storm-petrels along these
coasts could well reflect the substantive, regional environmental
gradation/variation that exists.
“So, the same phenomenon seen in Hydrobates
of the eastern North Pacific could very well apply to Oceanites in the
eastern South Pacific, i.e. for Oceanites oceanicus. (BTW, why do dark
storm petrels tend to frequent closer-to-coast waters than those having
substantial white? I’ve long wondered about that.)
“For the geographic distribution of
breeding areas for different Oceanites ‘species’ (as shown in the map
sent as part of the proposal) it could well be as much a result of species
variation as it is for humans not being able to cover the entire potential
breeding space, i.e. the Andes etc. from northern to southern, thus, to sample
the ‘in-betweens.’ At sea, these ‘species’ are mostly inseparable, except for a
few, e.g. gracilis (once upon a time Elliott’s SP), in part owing to the
likely broad overlap of Oceanites-types. So, what’s the point of trying
to recognize all these ‘species,’ especially on the basis of the very small
sample sizes (1-3???) thus far used for measurements and genetics? Golly,
ornithologists from yester-year should well be turning in their graves, given
all the effort they put out in their careers to recognize species variation as
much as they did, measuring countless specimens, but then along comes someone
with one specimen (3?) that somehow represents a new species based on DNA, although
it can’t really be separated, by eye, from others in the region?”
Comments from Robbins: “1. NO. I
just read through this again, including all comments. Not having sequenced the holotype is a red
flag that the authors are apparently attempting to rectify -- perhaps ask them
if that has been done. Moreover,
comments by Marín regarding measurements and correctly ageing the holotype also
cause one to pause in recognizing this taxon. So, for now, I vote no for recognizing
barrosi as a species.
“2. YES. Although sample sizes are
limited and the phylogenetic data are based solely on cyt b, I'm ok with recognizing
the three subspecies in part 2 as species.”
Comments from Lane:
“1) NO. After reading the
description, I was struck by the risk taken in choosing a holotype that I feel
cannot confidently be assigned to the same population as the birds studied on
the water that are used to define the species--much as I fear happened in the
description of O. pincoyae. This is a dangerous practice and may result
in something similar to the Strix omanensis situation, which was a
circus. Perhaps collecting in Chile is bureaucratically complicated, but the
description of a new species should be done with the best practices observed,
rather than cutting corners, or the authors may end up with egg on their faces.
At the very least, the holotype should be molecularly determined to be the same
as the birds that were studied in the field to be certain that all represent
the same taxon!
“2) NO. Although I suspect that in the long run there will be sufficient
evidence to support splitting up the members of the O. oceanicus/glacialis
complex as suggested, I feel we haven’t yet reached the threshold of
information yet to do so, and the comments by Howell, Shirihai, and Ainley
further make me think that being conservative is the right move for now.
“3) NO to English names (given 2).”
Comments
from Zimmer:
“1) NO. I share the
concerns expressed by others regarding the holotype, but also have to question,
given the phenotypic variation I’ve seen in putative pincoyae at sea, as
to whether barrosi isn’t better treated as a subspecies of pincoyae.
“2) YES, although not
without reservations, based upon the comments from Howell, Shirihai, and Ainley
(whose comments particularly resonate with me).
Ultimately, I’m with Nacho and Mark in being persuaded that the genetic
data, imperfect as they might be on various fronts, still point to recognizing
these taxa at the species-level, even if we can’t necessarily identify them as
such in the field!
“3) Depending on
whether or not Parts 1 & 2 pass, then “YES to the proposed English names,
although I must admit to not being wildly enthusiastic over “Graceful or
Gracile” for O. gracilis, not that I have any better ideas off the top
of my head. The other suggested names
seem fine to me.”
Comments from Remsen: “The
author team has done a great job elucidating this fascinating system given the
restricted information we have so far.
When this all gets worked out, it’s going to be quite a fascinating
story of speciation in progress, one that should attract substantial media
attention. However, had I been advising
them, I would have recommended publishing all these preliminary details separately
in a journal with a broader focus. This
would have set the stage for producing the additional crucial data that would
generate a stronger case for proposing taxonomic changes in a Zootaxa
paper. In fact, I hope that by the time SACC
processes this proposal there will be sufficient data ready to publish so that
we can more thoroughly evaluate the taxonomic aspects of their findings. Although my votes are negative, that strictly
refers to making a taxonomic decision at this point. This is somewhat unfortunate because my
overall feelings towards the analyses in the paper and its biological interest
is strongly positive. So, with that, my
votes are as follows:
“1). NO. Until genetic data
establish that the type specimen of barrosi is the same taxon as what
they are calling barrosi in the field, especially given the fine
distinctions in phenotypic. characters.
In fact, I am surprised that this was not caught during the review
process at Zootaxa because this is poor taxonomic practice, and one that
could be quite embarrassing to the authors if by chance it turns out to be a
problem. I’m hoping that this is not the
case and that this step in the process turns out to be just a formality.
“2). NO. I suspect that the authors
are correct in their assessment of species limits in this group, but that is
not a sufficient reason for me to endorse a major change in species
limits. The authors point out the gaps
in knowledge in terms of voice and smell (good idea) that would need to be
filled before making a formal decision on species limits. Note also that not one taxon is diagnosable
based on morphometric data (the PCA analysis), so we are left with subtle,
variable plumage characters. Although
the time-calibrated tree strongly suggests recognizing different species simply
on lineage age (in my opinion), the genetic data are based entirely on
cytochrome-b, and so I do not think that the estimates of lineage ages should
be taken seriously until a broader sampling of genes is available. At this point I would consider the genetic
data suggestive but not conclusive. The
mitochondrial gene tree is certainly consistent with what a species tree should
look like, but I think for the authors’ own protection, so to speak, some nDNA
and perhaps more samples are needed before formalizing this taxonomically.
“3). NO. Some of the names are
terrific but some could use another look, so I don’t like the idea of an all or
none package on the names. If the
proposal passes, then I think the authors would benefit from a 1x1 scrutiny of
each name. For example, as pointed out
by Nacho, “Andean” Storm-Petrel seems a little broad given its tiny
distribution, and “Graceful” seems a little contrived. I’m not saying we could do better, but that’s
exactly why we do in-depth separate proposals on English names in cases like this.”
Comments from Claramunt: “YES.
Very difficult decision. This problem needs more collecting of specimens and
sequencing of informative nuclear loci. Some of the taxa involved don’t seem to
be diagnosable. Mitochondrial lineages tend to sort geographically which may or
may not be indicative of independence of lineages. In particular, birds from
the same locality may have the same mtDNA haplotype, and sample size and
geographic coverage is so low that every taxon may appear monophyletic just by
chance. However, I am persuaded by the fact that in the few cases of sampling
localities that are far apart, mtDNA lineages still match taxonomy. That is the
case for O. exasperatus, with samples all around Antarctica, with some
populations closer to South American populations, yet monophyletic. At a
smaller scale but relevant, gracilis samples are taken from three
localities that are far apart, spanning the species distribution, yet they form
a clade and the samples of barrosi show up in the sister clade, despite
proximity to the southernmost samples of gracilis. Therefore, a picture
emerges in that this complex is indeed formed by multiple reproductively
isolate species-level lineages. We already recognize pincoyae; we need to
recognize the other taxa too; otherwise, the classification would totally
misrepresent relationships. It is unfortunate that he holotype of barrosi
is a bit atypical in plumage and has not been sequenced yet, but I prefer to
take the risk and recognize the new species as opposed to ignore the existence
of a species for purely nomenclatorial problems.”
Comments from John Croxall (voting
for Del-Rio): “From all this [an in-house analysis for BLI], my response to
the questions is as follows:
“A) NO to recognizing barrosi at any rank, but noting that
this is largely influenced by the information provided by scientists much more
familiar with the Fuegian taxa than I am.
B1) YES to recognizing chilensis as a monotypic species but
noting that I would defer to those with specific knowledge of this taxon.
B2) NO to recognizing exasperatus at any rank other
than a weak subspecies of O. oceanicus. This is an unqualified view,
based on considerable personal experience with O. oceanicus.
B3) NO to recognition of galapagoensis as a species on the
basis of the data provided in the Norambuena et al. paper.
C) Fuegian Storm-petrel for O. chilensis; N/A for rest.”
Additional comments from Manuel
Marín: “I would like
to add a minor comment. The sampling
localities for the genetic analysis are misleading for exasperatus. The
genetic samples are from the Indian Ocean, whereas the samples for morphometric
are Atlantic or Pacific oceans. The
genetic samples are from the other side of Antarctica (Indian Ocean),
and they have a map in the publication as if the genetic samples were from the
Antarctic Peninsula, which is closest to South America. Indian Ocean and
Atlantic or Pacific populations obviously have been isolated from each other
for a long time, and that probably what it makes them genetically slightly
different.”
Additional comments from Claramunt (response
to Manuel): “Sequence JN587565 is
listed as from Cape Hallet (E Antarctica-Pacific Ocean) in Pacha et al. (2020),
but the sequences seem to be from King George Island, South
Shetlands, according to the original paper (Robertson et al. 2011). Sample KU217327 (from Wallace et al.
2017) is from Livingston Island (also Shetlands). Therefore, these two samples are
from the tip of the Antarctic peninsula, “only” 1,000 km from the tip of South
America, but are clearly part of an exclusively Antarctic clade (exasperatus)
that is not mixing with the South American forms. Unless I’m missing something,
this supports the interpretation in Norambuena et al. and my previous comment.
“Regarding the type locality of oceanicus,
it is not unclear at all. The type locality is the locality where the type
species came from: the mouth of the Rio de la Plata. But, yes, there are two
problems: 1) the type specimen itself seems to be lost forever. 2) the breeding
site/population of the type is unclear. Solving this problem will require
designating a convenient neotype, and picking a specimen from the
Falklands/Malvinas would make a lot of sense, as they don't have a name
already, seem well differentiated from others, and they are the closest to the
original type locality.”
Additional comments from Manuel Marín (response to Santiago): “Santiago, ok, mi error, pero trate de referirme
a las poblaciones del Pacífico oriental y del Atlántico occidental. Sí, tienes
razón, pero creo que solo hay UNA muestra segura de las islas Shetland del Sur,
el de la isla Livingston. Una de N Caroline, EE. UU. Una que teniendo la misma muestra indican
diferentes localidades para el mismo tejido. Pondría eso en duda cual fue el
sitio original; bueno, aquí hay que revisar la muestra original, no el
artículo. Aunque la muestra JN587565 de la Universidad de Otago,
tiene más sentido que sea del cabo Hallet (que está en la parte más
sudoccidental del Océano Pacífico, cerca del Océano Índico (en la entrada norte
del Mar de Ross). En el cabo Hallet, hace unos años había una estación de NZ
que fue desmantelada, hoy en día solo hay (un refugio estacional de Nueva
Zelanda). Por mi experiencia la especie
no es rara en esa área, particularmente entre el cabo Hallet, las islas Possession y el cabo Adare.
“Desafortunadamente, no tengo esos artículos que mencionas, ¿el correcto
sería Pacha et al. (2020) como mencionas o Pacha et al. (2023) como se cita en
el artículo? Sobre los individuos de
áreas antárticas sí, estoy de acuerdo en que es una subespecie diferente, pero
no una especie. Y esa idea se remonta a la década de 1960, con Murphy o tal vez
antes. Sin embargo, Beck y Brown (1972)
la ubican muy bien, indicando que no hay razón para colocar más de dos
subespecies zonales para Wilson. Las dos subespecies serían O. o. oceanicus,
que comprenden las poblaciones al norte de la Convergencia Antártica o Frente
Polar. Lo que incluye poblaciones de
áreas como las Malvinas/Falklands y el sur de América
del Sur y probablemente Crozet Is.
Y el segundo sería O. o. exasperatus, que serían poblaciones al sur de
la convergencia o Frente Polar, que incluye las poblaciones de Georgia del Sur,
Orcadas del Sur, Península de Ant. y las áreas al sur
del Frente Polar alrededor de la Antártida.
Lo cual también tiene más sentido. Ellos (Beck y Brown) indican que se
trata de una separación que refleja diferencias adaptativas en morfología,
reproducción y alimentación y que están asociadas con áreas de clima diferente
o una zona biótica diferente. Muchos han seguido esa forma solo con dos
subespecies para Wilson SP.”
Comments from David Vander Pluym
(voting for Stiles): “Interesting proposal and discussion. I certainly think there
are more species in this complex than are currently recognized and the author
team does a good job of trying to sort this out, but they/we are still dealing
with limited data. The genetics are based only on Cytb so we have a gene tree
rather than a species tree, which can be informative but I’m not sure it is
telling the whole story here. With just Cytb and without any information on
vocalizations which are important in species recognition I don’t think we have
enough to fully inform taxonomic changes. Given the difficulties in finding
storm-petrel nests I also wonder if these populations are as isolated from each
other as we think. With the difficulties in getting vocal data, I wonder if we
might be better off doing studies on the chemical compositions of the
uropygial glands, but I digress. Overall, I think the authors have brought
up some really interesting hypotheses, but I do not think we are quite there
yet to make taxonomic changes. I could be convinced if more than just the mtDNA
was sampled, but with just a gene tree I don’t find the rest compelling enough
without more information for the splits at this time.
“1. NO. As others have
pointed out, the holotype has not been sequenced and there is a question about
its age. While I find the plumage differences discussed to be interesting, I do
find the analogy with Leach’s Storm-Petrel to be compelling. At this time there
is not enough evidence (in my opinion) that this is a biologically separate
species from pincoyae and potentially the two are separate from gracilis.
“2a. NO (for now) to
split chilensis. I am on the fence with my vote on this one, as it has
long been suspected to be a separate species, and I think further evidence will
likely confirm this. However, given the surprising results from the Cytb tree
with chilensis being sister to the rest of the complex I want further
confirmation that this is the correct placement before I feel a taxonomic
change can be made.
“2b. NO to split exasperates.
As others have pointed out, the Falklands are quite different from true
subantarctic islands, and it is possible that this is actually an undescribed
taxon rather than representing oceanicus.
“2c. NO (for now) to
split galapagoensis. As with chilensis I am on the fence with
this one; again, it has previously been split and likely is a separate species.
Again, however, I feel the need to be cautious given the surprising results
from the gene tree. This could be correct and if galapagoensis is sister
to oceanicus then a split is needed (but again were true oceanicus
sampled?), but I think being conservative is best at the moment for these
splits.
“3. NO given my no
votes above. Although I do like Andean and Fuegian, others likely would need a
separate proposal as there are other names in use for them.”
Additional comments from John
Croxall:
“Leading
experts (Ainley, Howell, Shirihai, Marin) have already recognised
fundamental flaws, which in their view preclude recognition of barrosi.
“See
below (Annex 2) my assessment of taxonomic issues relating to Oceanites
oceanicus, to which I have appended a paragraph on one implication of the
Norambuena et al. paper.
“I
discussed this and the paper briefly with Prof, Petra Quillfeldt
(who works, inter alia, on prions and storm petrels in the Falkland
Islands). She undertook a quick re-evaluation of the genetic data, having
identified some methodological flaws and observed:
“We
had a quick check of the sequences in Norambuena. There are a few problems and
inconsistencies, e.g.
• “the text mentions 40 samples, but the Table and
Figure we see only 28
• “the alignment used is over 1000 base pairs long,
but this is not the overlapping region, which in fact is only ca. 260 bp long.
• “when the overhangs are trimmed (which should have
been done), the resulting tree still shows Falkland WSP separate from Antarctic
WSP (97% support in the rough analysis done), but all support values within the
Falkland/pincoyae/galapagoensis/gracilis group are low --> more data
are needed here.
• “barrosi is not a separate species according
to this (and anyway, based on one pincoyae sample this is a bold claim
...)”
“Her
revised version of the Norambuena tree is attached (Annex 1 below), which shows
different (and much-reduced) support values at many of the key nodes.
“My
conclusions from all this include:
“1. Although the
genetic differentiation only has c. 84% support, it is probably safe to recognise O. chilensis Mathews, 1934 as a valid
species (Fuegian Storm-petrel).
“2. The Falklands
(Beauchene) population is genetically distinctive. It had previously been
assumed to belong in O. chilensis, but this is completely unsupported
genetically, its closest relatives being the other Chilean taxa. On
biogeographic grounds it is most unlikely to be con(sub)specific with nominate O.
oceanicus (type from [restricted to] South Georgia; population unsampled).
[Sampling is in progress to look into this]
“3. As no authentic
material of O. o. oceanicus has been sampled genetically, there is no
reason to change the status of O. o. exasperatus as a weak (clinal)
subspecies of O. oceanicus. However, for any authorities not recognising exasperatus, there is no good reason to
do so now.
“4. O. barrosi
is currently untenable at any rank, inter alia because: a) the type
specimen (whose status and provenance are uncertain- see Marin comments above)
is unsampled genetically; b) the 3 nominal barrosi specimens sampled assort very closely
genetically with the single specimen of O. pincoyae sampled. Moreover,
the distinctiveness of these 4 samples from the rest of the O. gracilis
complex has low (45%) support. Pending further sampling and critical study,
treating barrosi as a synonym of pincoyae seems inevitable
(noting that the status of O. pincoyae itself might benefit from further
confirmation).
“5. The genetic data
provide essentially no (25 %) support for recognising
O. galapagoensis as a species distinct from O. gracilis, even
though this might be plausible on some other grounds.”
“Annex
1: Oceanites
tree from Norambueno et al. (2024) as revised by Prof.
Petra Quillfeldt:
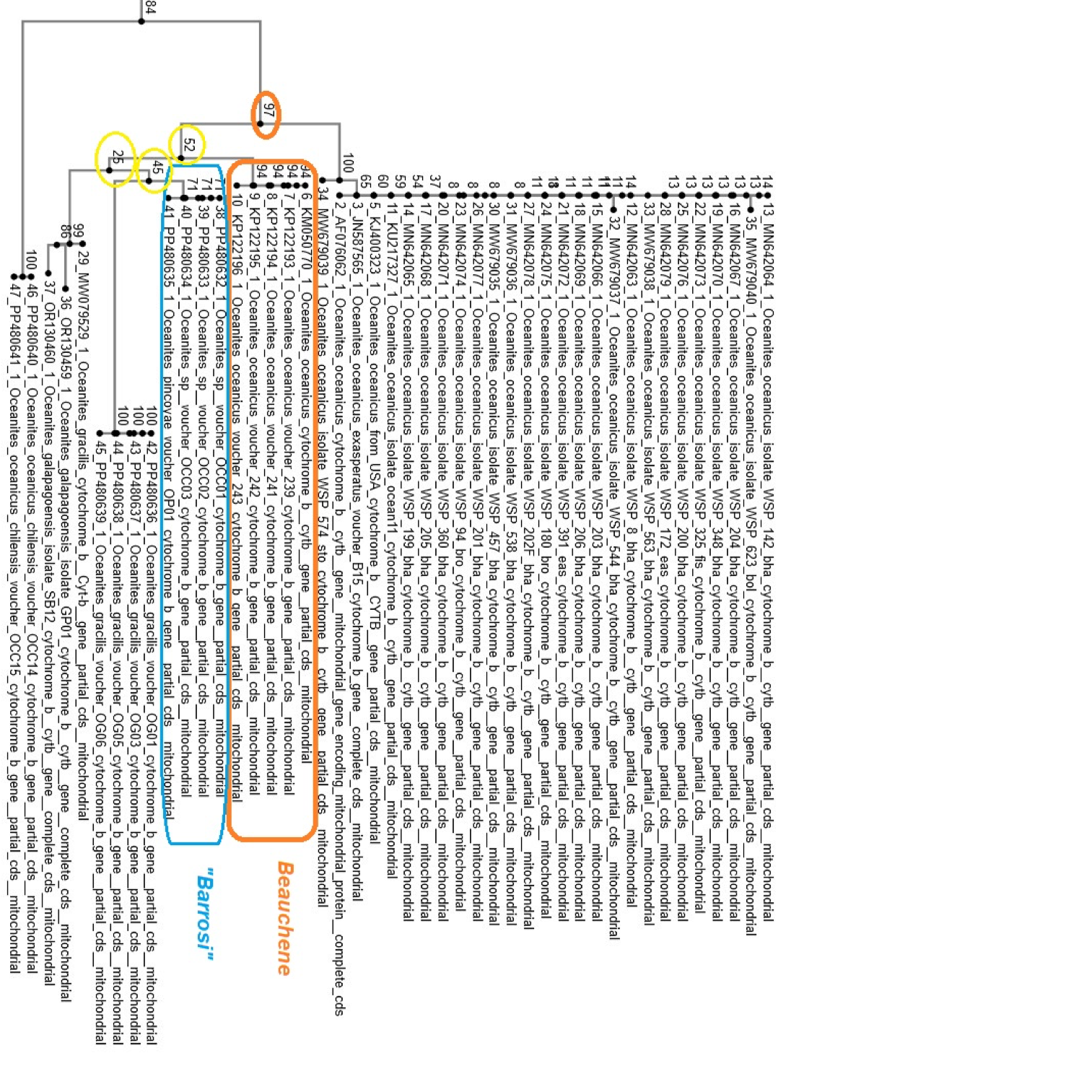
“Annex
2
“Wilson’s
Storm-petrel Oceanites oceanicus
“1. Described as Oceanites
oceanicus Kuhl, 1820, as noted by Mathews (1912) on the basis of a name and
illustration provided by Parkinson, itself based on a specimen collected off
the mouth of the Rio de la Plata,
Argentina, the type locality proposed by Mathews (1912). The original
description provided no further details, and the original specimen is no longer
extant (Roberts 1940). Thus, breeding provenance relating to the type specimen
of this name cannot be determined (even if the strong balance of probability is
that it derived from a population breeding in the Scotia Sea or adjacent
subantarctic/polar areas).
“2. Oceanites
oceanicus exasperatus was described by Mathews (1912), based on a type
specimen (# 244) from New Zealand seas or, more specifically, from “islands to
the south of New Zealand”. Mathews (1912) distinguished the “South Pacific”
populations it represented from the South Atlantic populations of the nominate
subspecies by their larger size (but gave no other details). The wing length of
the type (Rothschild collection, now in AMNH) was subsequently measured (by
Murphy; see Roberts 1940) as 155 mm. This is closely comparable to mean values
(153-155 mm; Roberts 1940) for populations breeding on the Antarctic Continent
south of New Zealand. Note further that Mathews (1912) expected that specimens
from the North Atlantic represented a separate taxon (breeding population), for
which the name wilsonii Bonaparte,1824 (type from Newfoundland) was
potentially available.
“3. Murphy (1918)
elucidated the interhemispheric migration of the South Atlantic populations of
the species (thus accounting for the records in
the North Atlantic and relegating wilsonii to the synonymy of
nominate oceanicus.) Curiously,
neither Murphy (1918) nor Murphy (1936) made any specific reference to O.
o. exasperatus as described by
Mathews (1912), despite criticising Mathews (in
Murphy 1918) for restricting the type locality of O. o. oceanicus to
“the mouth of the Rio de la Plata”.
Murphy (1918) noted that O. oceanicus bred at South Georgia and,
in his use of data from South Georgia, treated this as a de facto
restricted type locality for O. o. oceanicus. However, despite
influential subsequent publications (see, for instance, Marchant & Higgins
(1990) and Brooke (2004)) specifically
treating South Georgia as the type locality as restricted by Murphy
(1918)), I cannot locate a definitive statement by Murphy to this effect.
Notwithstanding, if South Georgia is not regarded as (or formally designated
as) the restricted type locality, the correct application of Kuhl’s name cannot
be determined, given that populations from several sources (Antarctica,
Antarctic Peninsula, South Georgia, Falkland Islands, Fuegia)
do, or might, occur in waters off north-east Argentina. Customary use would be
best served by confirming South Georgia as the type locality for Oceanites
oceanicus.
“4. Murphy (1936)
described O. o. chilensis, based on specimens from on or near breeding
grounds in Chilean Fuegia (specifically Wollaston I.
(type), Morton I. and Magallanes), based on small size (wing of type
series134-138mm). Thereafter (at least up to Brooke (2004) and OSNZ 2010)),
major works simply treated it as a synonym of the nominate subspecies. However,
it was resurrected as a subspecies by Onley & Scofield (2007) and OSNZ
(2022), and its nomenclature was reviewed by Palma et al. (2012a, b), who concluded
that the correct authority was Mathews, 1934, as a senior homonym (and with the
same type specimen) of O. o. chilensis Murphy, 1936. It was suggested as a possible species by
Howell (2012) and treated as such by Howell & Zufelt
(2019) and Harrison et al. (2022).
“5. Falla (1937)
described O. o. parvus from breeding birds at Kerguelen, based on small
size (wing length 142-144mm from Roberts (1940) and Marchant & Higgins
(1990)). Thereafter, this taxon has consistently been regarded as a synonym of
the nominate subspecies. However, Bretagnolle (1989) reported significant
differences in some vocalisations between birds from
Kerguelen and Terre Adelie (East Antarctica).
“6. Delimitation of the
breeding distribution of the two main subspecies of O. oceanicus is not
consistently represented. Typically, the nominate subspecies is taken to
comprise populations breeding at “subantarctic islands” north of the Antarctic
Polar Front (APF; formerly Antarctic Convergence) and O. o. exasperatus
as applying to all populations breeding to the south of the APF (e.g. Marchant
& Higgins 1990). However, South Georgia, which lies south of the APF, is
explicitly (Marchant & Higgins 1990) or implicitly (del Hoyo & Collar
2014) treated as nominate oceanicus (consistent with this being the
actual or potential type locality). There are no known plumage distinctions
between the two subspecies, so allocation depends on measurements.
“7.
The last comprehensive review of measurements was by Copestake
& Croxall (1985), whose tabulated data were included almost in full in
Marchant & Higgins (1990), who added new data for Kerguelen and Crozet
(both just north of the APF). A much-simplified summary, using mean wing length
(mm; range of sd is c. 2-4 mm) as the single most useful criterion, is:
Antarctic Continent: 152-155 (n=21)
Antarctic Peninsula: 152-153 (n=27)
South Orkney Is: 147-151 (n=82)
South Georgia: 149-153 (n=327)
Crozet Is: 143 (n=29)
Kerguelen: 142 (n=14)
Falkland Is: 137.5 (n=4)
South America (Fuegia):
134-138 (n=11) [Note this represents O. o. chilensis]
“My
main tentative conclusions are:
a) Moving
north from Antarctica to South Georgia, size variation is modest and
essentially clinal. There is no obvious reason not to treat South Georgia birds
as consubspecific with O. o. exasperatus, with
O. o. oceanicus having priority.
b) Subantarctic
Indian Ocean (Kerguelen, Crozet) birds are consistently slightly smaller than
subantarctic Atlantic Ocean (South Georgia) birds. The status (and range) of O.
o. parvus might usefully be reviewed (including and especially
genetically).
c) In
Atlantic populations there is a very significant discontinuity in wing length
(essentially no overlap) between South Georgia and Falkland Islands/Fuegian
populations, and thus these are unlikely to be consubspecific.
This is not surprising, given the very different marine ecosystems and avifauna
at South Georgia and the Falkland Islands.
“8.
The third point above is the most important. The new genetic data (Norambuena
et al. (2024) show that not only is topotypical O. o. chilensis highly
distinct (meriting species status) but that Falklands material is not
conspecific with either O. chilensis or O. o. exasperatus (material
from Antarctica). The identity of South Georgia material is crucial for
understanding and resolving this enigma.
“References:
Bretagnolle, V. 1989.
Calls of Wilson's storm petrel: Functions, individual and sexual recognitions,
and geographic variation. Behaviour 111:
98–112.
Brooke, M. de L. 2004. Albatrosses
and Petrels across the World. Oxford University Press, Oxford.
Copestake, P.G. & Croxall,
J.P. 1985. Aspects of the breeding biology of Wilson’s Storm Petrel Oceanites
oceanicus at South Georgia. British Antarctic Survey Bulletin 66:
7-17.
del Hoyo, J. & Collar, N.J. 2014. HBW
and BirdLife International Illustrated Checklist of the Birds of the World.
Vol. 1: Non-passerines. Lynx Edicions, Barcelona.
Falla, R.A. 1937.
Birds. Reports of the British, Australian and New Zealand Antarctic Research
Exoedition, Series B 2: 1-304.
Harrison, P., Perrow,
M.R. & Larsson, H. 2021. Seabirds. The New Identification Guide.
Lynx Edicions, Barcelona.
Howell, S.N.G. 2012.
Petrels, Albatrosses and Storm-petrels of North America: A Photographic Guide.
Princeton University Press, Princeton & Oxford.
Howell, S.N.G. & Zufelt, K. 2019. Oceanic Birds of the World. A Photo
Guide. Princeton University Press, Princeton & Oxford.
Marchant, S. &
Higgins, P.J. 1990. Handbook of Australian, New Zealand and Antarctic Birds.
Volume 1: Ratites to Ducks. Oxford University Press, Melbourne.
Mathews, G.M. 1912. Birds
of Australia 2: 11.
Murphy, R.C. 1918. A
study of the Atlantic Oceanites. Bulletin of the American Museum of
Natural History 38: 117-146.
Murphy, R.C. 1936. Oceanic
Birds of South America. Macmillan/American Museum of Natural History, New
York.
Norambuena, H.V.,
Barros, R., Jaramillo, A., Medrano, F., Gaskin, C., King, T., Baird, K. &
Hernandez, C.E. 2024. Resolving the conflictive phylogenetic relationships of Oceanites
(Oceanitidae: Procellariiformes) with the description of a new species. Zootaxa
5486: 451-475.
Onley, D. &
Scofield, P. 2007. Field Guide to the Albatrosses, Petrels and Shearwaters
of the World. Christopher Hem, London.
OSNZ 2010. Checklist of the Birds of New Zealand, Norfolk and
Macquarie Islands and the Ross Dependency, Antarctica. Fourth Edition. Te Papa Press, Wellington.
OSNZ 2022. Checklist
of the Birds of New Zealand. Fifth Edition. Ornithological Society of New
Zealand, Wellington (online only).
Palma, R.L., Tennyson,
A.J.D., Gaskin, C.P. & Jaramillo, A. 2012a. The scientific name, author,
and date for the “Fuegian storm-petrel”, a subspecies of Oceanites oceanicus
from southern South America. Notornis 59: 74-78.
Palma, R.L., Tennyson,
A.J.D., Gaskin, C.P. & Jaramillo, A. 2012b. A correction to Palma
et al. (2012) on the nomenclature of the Fuegian storm-petrel, Oceanites
oceanicus chilensis. Notornis 59: 178-179.
Roberts, B. 1940. The
life cycle of Wilson’s petrel Oceanites oceanicus (Kuhl). Scientific
Reports of the British Grahamland Expedition 1:
141-194.”
Additional comments from Remsen: “Here are
screenshots of Tom Schulenberg’s piece on all this from his “Splits, Lumps, and
Shuffles” column in Neotropical Birding 2025:”



