Proposal (1044) to South American Classification Committee
Treat Formicarius destructus as a separate
species from F. nigricapillus
Effect on SACC list
If
this proposal passes, it will result in the elevation of the taxon destructus to full species, resulting in
a monotypic F. nigricapillus and a
monotypic F. destructus.
Background information
The
current SACC note reads”
"1c.
Areta & Benítez Saldívar (2025) provided vocal evidence for treating South
American destructus as separate species.
SACC proposal needed."
Areta & Benítez Saldívar (2025) summarized the
situation as follows:
The
Black-headed Antthrush (Formicarius
nigricapillus) includes two allopatric subspecies: nominotypic nigricapillus in Costa Rica and Panama,
and destructus in Colombia and
Ecuador (Ridgway 1893; Hartert
1898; Wetmore 1972; Krabbe and Schulenberg 2003, 2020). Although the taxon nigricapillus
was originally described as a full species by Ridgway (1893) and treated as
such by some authors (Chapman 1917, Cory & Hellmayr 1924), it was often
considered as a subspecies of the Black-faced Antthrush (F. analis) (Hartert 1902, Ridgway 1911). Conversely, the taxon destructus was originally described as a
subspecies of F. analis by Hartert
(1898) and was subsequently either considered as such (Hartert 1902), rarely
afforded species-level status (Salvadori and Festa 1899; Howell and Dyer 2022),
or most often considered as a subspecies of F.
nigricapillus (Chapman 1917, Cory & Hellmayr 1924, Wetmore 1972, Krabbe
& Schulenberg 2003).
New information
In
Areta & Benítez Saldívar (2025), we assessed species limits in F. nigricapillus using vocal, plumage,
and morphometric data, concluding that nigricapillus
and destructus are better treated as
two separate biological and recognition species. For more detailed explanations
and discussions stemming from historical taxonomic references, please refer to
the publication. What follows is a blend of selected copied and reorganized
text and images from Areta & Benítez Saldívar (2025) with some minor
adjustments to facilitate reading.
Songs: After discarding
duplicates, Areta & Benítez Saldívar (2025) compiled a total of 57 songs of
nigricapillus and 129 songs of destructus that were assessed aurally
and through examination of spectrograms. The song of nigricapillus is a
rapid series of around 25 clear, pulsated whistled notes that begins with a few
more spaced notes that become mostly evenly paced with a slight rise in pitch
halfway through the song and a relatively monotonous ending (sigmoid-like
spectrographic contour; Fig. 1). The song of destructus is a very rapid eerie series of around 40 ventriloquial
notes that fall and rise in pitch and decrease markedly in pace in the second
half (smile-like spectrographic contour; Fig. 1). The vocalizations of nigricapillus
and destructus have been described
accurately in field guides, but the importance of their differences has not
been fully realized. The song of nigricapillus
was described as "a rapid, pulsating series of ca. 20 deep, resonant,
whistled notes, the first 2-3 slower, more staccato, the next 6-8 rising in
pitch, the last 10-12 on the same pitch, with the final 2 notes slower, the
entire series lasting 4-5 sec" (Stiles and Skutch
1989, p. 287), while that of destructus "resembles that of
Rufous-capped Antthrush, but shorter, an eerie, quavering glissando of about 30
notes in 3 sec, sliding upscale and slowing noticeably at the end;
ventriloquial" (Hilty and Brown 1986,
p. 417). The song of nigricapillus has been aptly likened to
the shorter song of Thicket Antpitta Myrmothera
dives ( Garrigues and Dean
2007; Vallely and Dyer 2018), a comparison that does not apply to the song of destructus.
The
quantitative acoustic characterization evidenced that songs
of both taxa are 100% diagnosable (n=21 nigricapillus,
n=38 destructus). Acoustic data showed that 13 out of 15 variables differed significantly
between nigricapillus and destructus, the differences in 10 of
these 13 variables were very marked with non-overlapping mean ± SD,
automatically indicating that they belong to statistically different
populations. Taxon nigricapillus
showed lower song peak frequency, longer mean note duration and mean interval
between notes, fewer notes per song, slower pace, relatively even pace in the
first and second halves of the song, lower peak frequencies of first, median,
and final note, and longer duration of first, median, and final note. In
contrast, destructus had higher song
peak frequency, shorter mean note
duration and mean interval between notes, more notes per song, faster pace, a
marked deceleration in the second half of the song, higher peak frequencies of
first, median, and final note, and shorter duration of first, median, and final
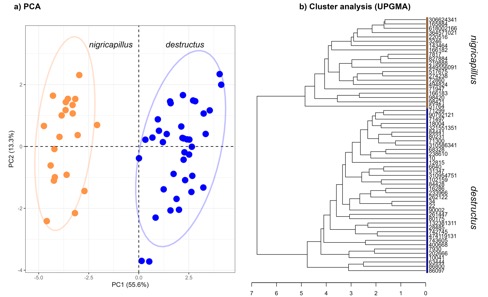
note (Table 1). The songs of both taxa were clearly separated in
multivariate space (Fig. 2A) and a cluster analysis also revealed two
distinctive groups, unambiguously including all destructus samples
clustered separately from all nigricapillus recordings (Fig. 2B).
The
song of nigricapillus remains
essentially identical through ca. 490 km across Costa Rica and into C Panama in
our sample. t]The song of destructus also
remains basically the same through its distribution, ca. 1130 km extending from
NC Colombia to SW Ecuador. The diagnostic song types are separated by a gap of
ca. 190 km between NW Colombia (Jardín Botánico del Pacífico, Chocó) and
E Panamá (Nusagandi, Guna Yala), and exhibit no sign
of intermediacy closer to their respective limits (Fig. 1). Further documented
records are needed to properly understand the actual distributional gap between
nigricapillus and destructus. The width of the gap is at
least 190 km as shown by our song-recordings dataset, but possibly much
smaller: birds seen, heard, and tape-recorded at Cuchilla del Lago on the
Colombian side of the Serranía de Darién in the Cerro Tacarcuna (Renjifo et al.
2017) most likely represent the taxon nigricapillus. This would reduce the gap
between nigricapillus (Cuchilla del
Lago; see question mark in Figure 1) and destructus
(Reserva La Bonga) to 100 km within Colombia. Unfortunately, the sound
recordings were not available at the time of our writing and could not be
assessed (J. Avendaño in litt.). Further fieldwork should clarify the extent of
their allopatry and assess whether the Río Atrato and its formidable swamps act
as a biogeographic barrier for these taxa (Haffer 1970, 1975, Renjifo et al.
2017). The Cerro Tacarcuna records, if confirmed to be nigricapillus (which seems very likely), would indicate that both
taxa (nigricapillus and destructus) occur in South America.
Fig. 1. Plumage
aspect, songs, and geographic distribution of
songs of Black-capped Antthrush (Formicarius
nigricapillus), and Black-hooded Antthrush (F. destructus) analysed in this study. Orange circles: F. nigricapillus (photo: ML-452131711, San
Gerardo Biological Station, Costa Rica, by
Mark Hebblewhite; song: ML-220516, Reserva Biológica Bosque Nuboso
Monteverde, Costa Rica by D. L. Ross).
Blue circles: F. destructus (photo: ML-121617911, Rio Silanche Bird
Sanctuary, Ecuador by Nick Athanas; song: JIA-10, Reserva de Bosque Seco Lalo Loor, Ecuador by J. I. Areta). Circles
with a central dot denote songs measured quantitatively; plain circles denote
songs studied aurally (see Appendices 1 and S1); question mark (?) indicates
unconfirmed sound recording of nigricapillus
from Cerro Tacarcuna in Colombia. The differences in plumage (chestnut nape in nigricapillus, black nape in destructus), song, and morphometrics
collectively support the recognition of nigricapillus
and destructus as separate species.

Table 1. Acoustic parameters
of songs of Black-capped Antthrush (Formicarius
nigricapillus), and Black-hooded Antthrush (F. destructus). Values shown are mean ± SD [range], n= sample size.
Asterisk denotes significant statistical differences in the Mann-Whitney
non-parametric test (α<0.05), and plus symbol denotes non overlapping mean ±
SD. See Appendix 1 for measured sound recordings.
|
Variable |
F. nigricapillus (n=21) |
F. destructus
(n=38) |
|
Bandwidth 90% (Hz) |
205.35±56.37 [93.8-281.2] |
207.93±38.45 [140.6-281.2] |
|
Duration 90% (s)* |
2.38±0.33 [1.74-3.00] |
2.64±0.44 [1.92-3.88] |
|
Peak frequency (Hz)* + |
1484.37±61.75 [1406.2-1593.8] |
1689.91±61.7 [1546.9-1781.2] |
|
Mean note duration (s)* + |
0.06±0.01 [0.05-0.09] |
0.04±0.01 [0.02-0.07] |
|
Mean interval between notes (s)* + |
0.08±0.01 [0.05-0.10] |
0.04±0.01 [0.03-0.06] |
|
Number of notes*+ |
24.95±1.69 [22-29] |
37.62±5.57 [28-52] |
|
Sound density |
0.56±0.06 [0.46-0.67] |
0.6±0.08 [0.47 - 0.79] |
|
Pace*+ |
5.98±0.42 [5.44-6.96] |
9.61±1.09 [7.76-11.81] |
|
Pace change (second/first half)* + |
1.04±0.09 [0.88-1.19] |
0.76±0.07 [0.63-0.93] |
|
(8.63±1.2/11.37± 1.08) [5.61-6.67/5.25-7.58] |
(6.18±0.32/5.99±0.66) [6.82-11.46/9.02-12.82] |
|
|
Peak frequency first note (Hz)* + |
1345.97±68.13 [1218.8-1500] |
1650.24±93.23 [1406.2-1781.2] |
|
Peak frequency median note (Hz)* + |
1412.91±47.54 [1312.5-1500] |
1617.82±58.76 [1453.1-1781.2] |
|
Peak frequency final note (Hz)* + |
1477.67±60.46 [1406.2-1593.8] |
1703.12±69.11 [1546.9-1875] |
|
Duration first note (s)* |
0.04±0.02 [0.01-0.06] |
0.02±0.01 [0.01-0.03] |
|
Duration median note (s)* + |
0.09±0.02 [0.04-0.14] |
0.12±0.03 [0.04-0.10] |
|
Duration final note (s)* |
0.14±0.04 [0.10-0.26] |
0.11±0.03 [0.06-0.21] |
*=non-parametric Mann-Whitney test P-value <.05
+=non-overlapping mean ± SD
Plumage: To characterize the
external appearance of nigricapillus
and destructus, Areta & Benítez
Saldívar (2025) examined photographs of 37 museum
specimens, including the holotypes of both taxa, and vetted 40 good-quality
photographs on the citizen science platforms eBird (ebird.org) and iNaturalist
(inaturalist.org). They found that nigricapillus exhibits chestnut-brown
hindneck (sometimes extending to neck sides in a handkerchief or semicollar; Figure 1), and typically more chestnut-brown
back (Fig. 1), whereas destructus exhibits all-black hindneck and neck
sides (Fig. 1), and typically browner back. There is some variation in back
colour of specimens (but not in the back-neck contrast that distinguishes
taxa), with some nigricapillus being
seemingly identical in colour to typical destructus
(Chapman 1917,
USNM specimens). In some nigricapillus
individuals, the chestnut-brown hindneck is extensive and expands onto the
sides of the neck creating a semicollar, which gives
these individuals a capped aspect, that is less prominent in birds with less
extensive chestnut-brown hindneck. On the other hand, all individuals of destructus show a hooded aspect, caused
by its wholly black head, hind neck and neck sides (Fig. 1).
Fig. 2.
Quantitative analyses of songs of Black-capped
Antthrush (Formicarius nigricapillus),
and Black-hooded Antthrush (F. destructus). (A) Plot of the first two principal components (PC1 vs.
PC2) of the Principal Component Analysis. Ellipses depict 95% confidence
intervals. (B) Dendrogram from the agglomerative hierarchical
cluster analysis (UPGMA). Both methods consistently
show that nigricapillus and destructus differ markedly in songs
supporting their treatment as separate species. See Appendix 1 and S1 for songs
measured.
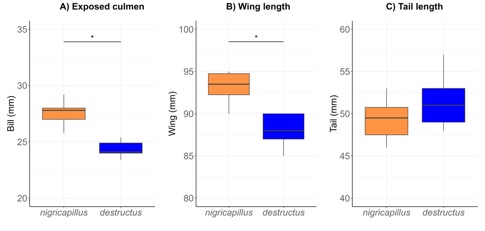
Morphometry: based on a limited
dataset (n=6 nigricapillus, n=9 destructus), Areta & Benítez
Saldívar (2025) concluded that 1) bill length (exposed culmen) showed no
overlap between taxa, with all individuals of nigricapillus having a longer bill than destructus, and therefore no overlap in mean ± SD values (Fig. 3a),
2) nigricapillus was longer winged
than destructus, with exact overlap
only in their extreme values, and no overlap in mean ± SD values (Fig. 3b), and
3) tail length was longer in nigricapillus
than in destructus, but the
difference was not statistically significant (two tailed t-test p=0.17) (Fig.
3c).
Fig. 3. Morphological measurements of Black-capped Antthrush (Formicarius nigricapillus), and
Black-hooded Antthrush (F. destructus).
Figure depicts median and quartiles on box plots. Asterisk denotes significant
statistical differences in one-way ANOVA (α<0.05). The
morphological differences in bill and wing length coupled to plumage
differences give support to the treatment of nigricapillus and destructus
as separate species. See Table 2 for sample sizes and data, and
Appendix 2 and S2 for specimens measured and studied.
The differences in songs and plumage herein
described can be readily appreciated in videos of free-ranging singing birds:
nigricapillus: https://macaulaylibrary.org/asset/608419951
destructus:
https://macaulaylibrary.org/asset/316228641
Taxonomic assessment
The
marked differences in vocalizations and morphology, and moderate but consistent
plumage differences strongly supports the elevation of the taxon destructus to species-level, leading to
the recognition of two allopatric and monotypic species, F. nigricapillus and F.
destructus. Areta & Benítez Saldivar (2025)
based their taxonomic conclusions on the recognition concept of species (Paterson 1985), whereas the same species would be recognized by applying
the biological species concept (Mayr 1963; “isolation
concept” fide Paterson 1985). The inferred level of discontinuity between nigricapillus and destructus is of such a magnitude that presumably any other modern
species concept would recognise them as separate
species, whether based on mating or other important attributes, phenotypic
distinctiveness, presence of autapomorphies, or phylogenetic independence
(Cracraft 1983, Mishler & Brandon 1987, Gill 2014, Areta et al. 2019,
Winker 2021). Howell & Dyer (2022:27) wrote that "Differences in plumage and song indicate that Central
American nigricapillus and South American destructus (Choco
Antthrush) are best treated as separate species." A view that is amply
supported by Areta & Benítez Saldivar (2025).
In
terms of plumage, the differences between nigricapillus
and destructus (Fig. 1) would be
among the least conspicuous for two Formicarius
species-level taxa, and comparable to (although less obvious than) those
between F. moniliger and F. analis. They differ most notably by
the presence of a rufous-chestnut fore-collar below the black throat in moniliger, whereas the black throat
contacts the grey chest directly in the two subspecies groups of F. analis
(Howell 1994, Vallely and Dyer 2018). However, less obvious plumage differences
exist between the analis and hoffmanni subspecies groups within F. analis despite their noticeable vocal
differences (Howell 1994) which are compatible with species-level differences
in the genus (Krabbe and Schulenberg 2003, van Dort et al. 2023, Benítez
Saldívar & Areta in prep.).
Common names
Most
species in the genus Formicarius
carry common English names that refer to plumage features. We propose to adopt
the name Black-capped Antthrush for F.
nigricapillus and Black-hooded Antthrush for F. destructus, which focus on one of the main plumage differences
between them and retain a connection to the former Black-headed Antthrush used
for the composite species. We find the proposed use of Black-hooded Antthrush
for F. nigricapillus by Howell and Dyer (2022) to be misleading, as this taxon is capped rather than
hooded. The smaller bill of destructus
is difficult to appreciate in field conditions, and bill features do not seem
useful to coin common names here. Finally, Choco Antthrush has been proposed
for destructus (Howell and Dyer 2022); although a good name, there are other antthrushes in the
Choco, and it loses the connection to the former Black-headed Antthrush name.
Voting and
recommendations
Perhaps
the best thing to do is to vote on three different aspects of the proposal:
A) Species limits: We
recommend a YES vote to split F.
destructus from F. nigricapillus.
B) English names: We
recommend a YES vote to adopt the name Black-capped Antthrush for F. nigricapillus and Black-hooded
Antthrush for F. destructus
C) Presence of nigricapillus in Colombia. A lingering
question is whether the Cerro Tacarcuna records in Colombia should be
provisionally considered to be nigricapillus
until shown otherwise. For biogeographic reasons, we lean towards the
maintenance of nigricapillus in the
SACC list based on these records, as it seems unlikely for destructus to reach the Cerro Tacarcuna crossing the Atrato swamps.
We recommend a YES vote to keep F.
nigricapillus in the SACC list.
References
Areta JI, Depino EA, Salvador SA, Cardiff SW,
Epperly K, Holzmann I (2019) Species limits and biogeography of Rhynchospiza
sparrows. Journal of Ornithology 160:973-991
Areta,
J.I., Benítez Saldívar, M.J. (2025). Species limits in the Black-headed Antthrush (Formicarius
nigricapillus). J Ornithol
https://doi.org/10.1007/s10336-025-02265-5
Chapman
FM (1917) The
Distribution of Bird-Life in
Colombia. A Contribution to a Biological Survey of. South America.
Bull. Amer. Mus. Nat. Hist., 36.
Cory CB, Hellmayr CE (1924) Catalogue of
Birds of the Americas. Publications of the Field Museum of Natural History
(Zoological Series). Volume 13, Part 3. Chicago.
Cracraft J (1983) Species concepts and
speciation analysis. Curr Ornithol 1:159–187
Garrigues R, Dean R
(2007) The Birds of Costa Rica. Zona Tropical, Comstock Publishing
Associates, Cornell University Press.
Gill F (2014) Species taxonomy of birds:
which null hypothesis? Ornithological Advances 131: 150–161
Haffer J (1970)
Geologic-climatic history and zoogeographic significance of the Urabá region in
northwestern Colombia. Caldasia 10: 603–636.
Haffer J (1975) Avifauna of
northwestern Colombia, South America. Bonner Zoologische Monographien 7: 1–182.
Hartert E (1898) On a
collection of birds from north-western Ecuador, collected by Mr. W. F. H.
Rosenberg. Novitates Zoologicae 5: 477–505.
Hartert E (1902) Some
further notes on the birds of north-west Ecuador. Novitates Zoologicae 9: 599–617.
Hilty SL, Brown WL (1986) A
Guide to the Birds of Colombia. Princeton: Princeton University Press.
Howell SNG (1994) The
specific status of black-faced antthrushes in Middle America. Cotinga 1: 20–25.
Howell SNG, Dyer D (2022). Costa Rica
even richer than we thought? North American Birds 73:
22–33.
Krabbe NK, Schulenberg TS (2003).
Black-headed Antthrush (Formicarius nigricapillus). In Handbook of the
Birds of the World Alive (del Hoyo J, Elliott A, Sargatal J, Christie DA, de
Juana E, eds). Lynx Edicions, Barcelona.
Krabbe NK, Schulenberg TS (2020). Black-headed Antthrush (Formicarius nigricapillus), version 1.0. In Birds of the World (del
Hoyo J, Elliott A, Sargatal J, Christie DA, de Juana E, eds). Lynx Edicions,
Barcelona.
Cornell Lab of Ornithology, Ithaca, NY, USA. https://doi.org/10.2173/bow.blhant1.01
Mayr E (1963) Animal species
and evolution. Harvard University Press, Cambridge
Paterson HEH (1985) The
recognition concept of species. In: Vrba ES (ed) Species and speciation.
Transvaal Museum Monograph, Pretoria.
Mishler BD, Brandon RN
(1987) Individuality, pluralism, and the Phylogenetic Species Concept. Biology
and Philosophy 2: 397–414
Renjifo LM, Repizo A, Ruiz-Ovalle JM, Ocampo S, Avendaño JE (2017) New
bird distributional data from Cerro Tacarcuna, with implications of
conservation in the Darién highlands of Colombia. Bulletin of the British
Ornithologists´ Club 137: 46–66.
Ridgway R (1893) A revision
of the genus Formicarius Boddaert.
Proceedings of the United States National Museum 16: 667–686.
Ridgway R (1911) The Birds
of North and Middle America: A Descriptive Catalogue. Part V. Bulletin of the
United States National Museum 50.
Salvadori
T, Festa E. 1899. Viaggio del Dr. Enrico
Festa nell’Ecuador. XXI. Uccelli. Parte seconda - Passeres clamatores.
Bollettino dei Musei di Zoologia ed Anatomia Comparata della R. Università di
Torino 15 (362): 1–34.
Stiles FG, Skutch AF (1989) A
Guide to the Birds of Costa Rica. Ithaca, NY: Cornell University Press.
Vallely AC, Dyer D
(2018) Birds of Central America. Princeton University Press, Princeton
and Oxford.
van Dort J, Patten MA,
Boesman PFD (2023). Black-faced Antthrush (Formicarius analis), version
2.0. In Birds of the World (S. M. Billerman, Editor). Cornell Lab of
Ornithology, Ithaca, NY, USA. https://doi.org/10.2173/bow.blfant1.02
Wetmore A (1972). The
Birds of the Republic of Panamá. Volume 3. Passeriformes: Dendrocolaptidae
(Woodcreepers) to Oxyruncidae (Sharpbills). Smithsonian Miscellaneous
Collections 150(3). Smithsonian Institution, Washington, D.C., USA.
Winker K (2021) An overview of speciation and species limits
in birds. Ornithology 138: 1–27
10.1093/ornithology/ukab006
Links to supplementary
information:
Addendum:
Genetics: The coincidental
break in plumage and vocalizations between F.
moniliger and F. analis umbrosus
(a representative of the hoffmanni
group) were used as arguments to support the elevation of moniliger to species status (Howell 1994). More recently, phylogenetic data have shown that F. moniliger is sister to a clade
including F. destructus as more
closely related to F. analis
(including representatives of the hoffmanni
and analis subspecies groups) (Harvey et al. 2020). This distant relationship between moniliger and analis
further reinforces the species-level split of F. moniliger and lends support to vocal differences as a useful
tool to establish species limits in Formicarius.
Although there are no available genetic data for nominotypic nigricapillus, the vocal distinctions
between nigricapillus and destructus seem as or more marked than
those between F. moniliger and F. analis of the analis and hoffmanni
subspecies groups. In our paper we predicted that nigricapillus and destructus
will exhibit levels of genetic differentiation tantamount to their vocal and
morphological distinctions.
The
Formicarius phylogenetic tree from
Harvey et al. 2020:

Harvey
MG, Bravo GA, Claramunt S, et al. (2020) The evolution of a tropical
biodiversity hotspot. Science 370: 1343–1348.
Juan
I. Areta and M. Juliana Benítez Saldívar, April 2025
Voting chart: https://www.museum.lsu.edu/~Remsen/SACCPropChart1044+.htm
Comments
from Remsen:
“A. YES. Although
playback trials would be nice, the degree of difference in the songs is
consistent with species rank, and burden-of-proof falls on those who would
treat them as subspecies.
“B. MAYBE, for all the
good reasons outlined in the proposal, especially maintaining the connection
between old and new names. Also, follows
SACC guidelines (https://www.museum.lsu.edu/~Remsen/SACCEnglishNameGuidelines.pdf)
for providing new names for both daughters in a parental split. After having looked through Macaulay photos
of dozens of destructus, “hooded” seems especially appropriate for the
extent of the black. The match between
the scientific and English names for nigricapillus is helpful in
remembering both. However, as for nigricapillus,
a problem is that “capped” is typically used for cases in which the bird has a
much more distinctive color patch on the top of the head, which is not really
the case for nominate ruficapillus, despite the scientific name. Obviously, the scientific name reflects a
pattern seen on specimens, but I think we need side-by-side photos of the
specimens, if only as confirmation that the name is OK.
“C. YES, but somewhat
reluctantly because the recordings are unavailable and because perhaps the
Colombian national committee should have the say on this one. On the other hand, to propose that the
records are not nigricapillus makes no biogeographic sense, and I agree
with this statement in the proposal “Cerro Tacarcuna records in Colombia should
be provisionally considered to be nigricapillus
until shown otherwise.”
“Anecdote: puzzled by
the name destructus, I looked at Jobling (online), and here is the
derivation from Hartert’s 1898 description: ‘very much destroyed by the shot, but still quite recognizable, is so
considerably smaller and darker brown above than typical analis that I
do not hesitate to distinguish it subspecifically’.”
Comments
from Lane:
“I must admit this one was not on my radar (although I have seen, and heard,
both taxa). The voices are much more distinct that I was expecting, and I think
the results of the study presented are good.
“A) YES to splitting
the two members of the complex as biological species.
“B) The two daughter
species are not all that strongly differentiated by plumage, and really can't
see F. nigricapillus having a distinctive black crown set off against
the rest of the head (I suspect the name was coined because it was
blacker-crowned than F. analis... and granted, I am looking at the
poorly-lit Macaulay photos rather than specimens to make this statement), but
unless we want to get derailed by another English name situation, I will go
with the names suggested: Black-crowned and Black-hooded.
”C) YES to retaining
both species in the SACC area due to the records from the border near Panama.”
Comments from Rasmussen
(who has Robbins’ vote): “B. Re the suggestion that the presence of other
antthrushes in the Choco makes Choco Antthrush for destructus less than
a great name, I don't see the issue there. As far as I can see, destructus is
the only antthrush in the majority of lowland Choco,
and certainly it is the only one recognized as a species that is largely
confined to the Choco (unless I'm missing something---please correct if so).
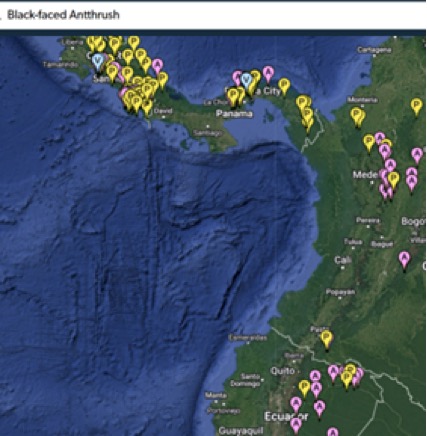
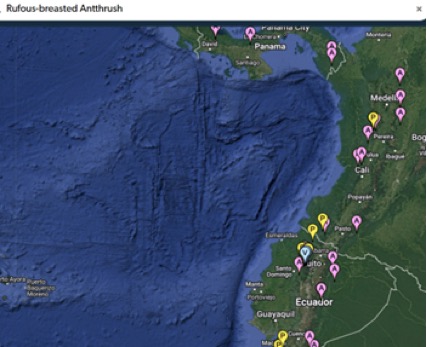
Here are maps of the three other species in the region:
“There are a smattering
of Black-faced eBird reports for the Choco but no photos.
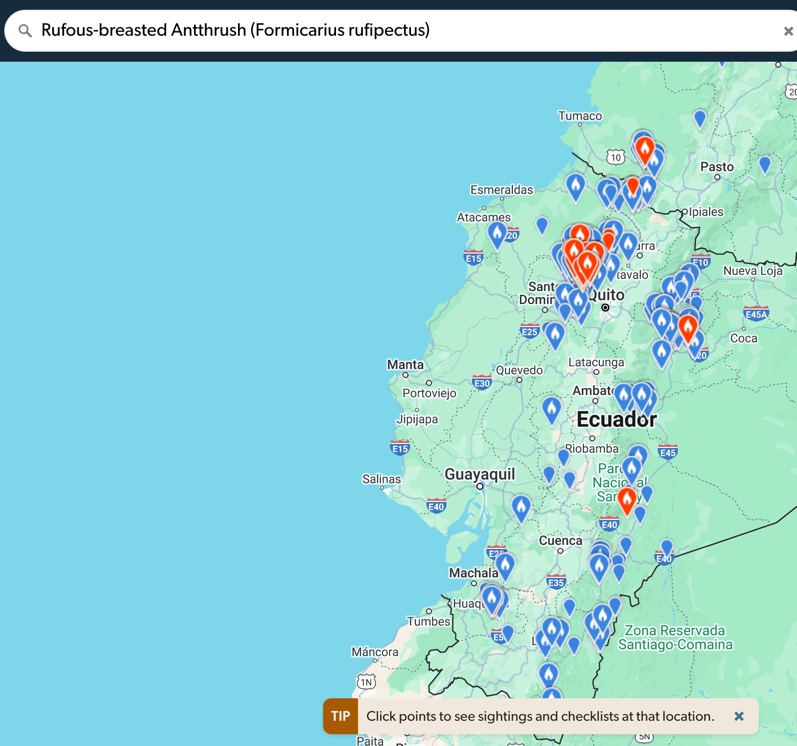
“Rufous-breasted is in
the western Andes but not lowland Choco:
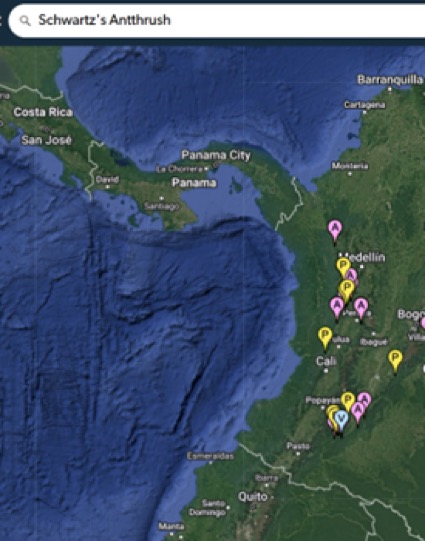
“Schwartz's Antthrush
is also in the Andes, not the lowlands.
“All the others
currently called antthrushes are either eastern slope or nowhere near the
Choco.
“Thus, I'm voting for
Choco Antthrush for destructus. I think Black-hooded is OK too.
“In the absence of
something better, I'm still OK with Black-capped for nigricapillus,
especially given the sci name.”
Additional comments
from Remsen:
“Terry Chesser graciously sent me photos of the USNM specimens of nigricapillus
(1) and destructus (4). I have
lightened up the originals, and done some cropping and rearranging so that the
specimen of nigricapillus is always at the top, above the yellow line:
“Dorsal:

“Lateral:

Ventral:

“As
noted by Terry, there does not seem to be much in the way of difference between
the two in plumage, especially in how “capped” the two are. Of course, with only 1 nigricapillus, it’s
tough to assess this, but I am backing off of the proposed English names and
going with the names used by Howell and Dyer (2022), which have the additional
advantage of already having been in print for 3 years. Also, as shown by Pam, destructus is “the”
Antthrush of the Chocó. As much as I
appreciate the logic of the proposed names, I am switching my vote on B to NO
unless additional information indicates that nigricapillus has a ‘capped’
appearance.”
Comments
from Terry Chesser: “If destructus
is more closely tied to the Choco than any other antthrush,
then I agree with Pam that Choco Antthrush is a perfectly good name. The name Black-hooded Antthrush, although
suggested in the proposal for destructus, is also applicable to nigricapillus,
and in my view is more appropriate for nigricapillus than Black-capped,
which despite matching the scientific name doesn't seem to be particularly
suitable (as others have noted). Black-headed or Black-hooded, yes, but not
Black-capped, at least in the way that "capped" is typically used. A bonus is that Black-hooded for nigricapillus and
Choco for destructus were used in 2022 by Howell and Dyer, so
there's a bit of precedence there.”
Additional
comments from Areta:
“I find hooded for nigricapillus a terrible name. Look at photos of live
birds in their habitat, and you´ll see how strikingly different is the hood of destructus
(which gives it a bold hooded appearance) from the brown nape (and sometimes
neck sides) of nigricapillus, which very often gives it a capped appearance
(and if one would like to call this hooded, then it is obviously much, much,
much less hooded than destructus, to the point that the name results in
confusion, because one would be calling hooded the presumed sister species that
is less hooded). Choosing a species name based on a feature that is more
prominent in its sister species seems to be a bad choice to me.
“Note
also that ALL the field guides (including the latest from Costa Rica by Howell
and Dyer) have erred, and they have illustrated destructus instead of nigricapillus.
“Here
some random nigricapillus photos:
https://macaulaylibrary.org/asset/622843058
https://macaulaylibrary.org/asset/622070151
https://macaulaylibrary.org/asset/613829664
https://macaulaylibrary.org/asset/601316591
https://macaulaylibrary.org/asset/452110921
https://macaulaylibrary.org/asset/153191961
“And
some destructus:
https://macaulaylibrary.org/asset/205559781
https://macaulaylibrary.org/asset/184627131
https://macaulaylibrary.org/asset/121617911
https://macaulaylibrary.org/asset/76928221
“Anyway,
I stand by the names we proposed in the paper.”
Comments
from Gary Rosenberg (voting for Claramunt): “I vote YES with regard to
Black-capped for nigricapillus and Black-hooded for destructus
- as it has been demonstrated on looking at the photos that Nacho provided that
nigricapillus is really visually more “black-capped” in comparison to destructus
– with destructus having a more extensive black hood. Although seeing
those differences in the field may be difficult. I personally would retain
“Black-headed” for nigricapillus, but I guess that isn’t an option - we could
use Black-headed and Choco Black-headed - or if retaining Black-headed is
really against the rules, then using Black-capped for nigricapillus would be
acceptable - it therefore would align with the scientific name. I am not
against the idea of using “Choco” for destructus, but I don’t believe it
is the only antthrush there - or at least it is a
matter of semantics. In western Ecuador,
“destructus” ALMOST overlaps with Rufous-breasted - the two are
separated by elevation, but I would NOT be surprised if the two overlap (and I
suspect they do overlap around Mindo) – nigricapillus and
Rufous-breasted overlap in elevation in Costa Rica (I have seen the two on the
same trail in Braulio Carrillo NP). Here is a more specific map of where
Rufous-breasted occurs in Ecuador - therefore the idea that destructus is
the only antthrush in the “Choco” is a bit misleading
- in my opinion. One can make the argument that the “Choco” is not limited
specifically to the lowlands but also includes the foothills. Furthermore, destructus
does occur south along the slopes of the Andes in Ecuador south to about
Guayaquil - south of the area generally described as the Choco - although the
majority of the distribution is within the Choco for sure.
“I
guess I prefer using Black-hooded for destructus, it is at least similar
to “Black-headed” and as the authors say, retains that connection, but I would
not have an issue with using Choco for destructus, as the majority of
the bird’s range is in the Choco.”
Additional
comments from Remsen:
“I am flip-flopping back to YES on this one after seeing the photos for which
Nacho provided the links. I am also
strongly persuaded by his excellent point that using “hooded” for nigricapillus
would be a bad idea given that “black-hooded” is more appropriate for its
sister taxon.”
Comments
from Josh Beck (guest vote):
“Essentially
there are two good and suitable and relatively unproblematic names for destructus
suggested/available (Black-hooded and Choco) and two slightly more problematic
and less well received names suggested/available for nigricapillus (Black-capped
and Black-hooded).
“Viewing
this pragmatically, I don't think any of the proposed names are really going to
cause any issues. This is not a group of birds where confusion reigns supreme
or where there is such a diversity of species that we're entering into name
soup. Formicarius are almost universally first detected and ID'd by voice and overlapping species are different enough
from each other that subtle details of names aren't going to impact visual
field ID either. Nor do I think someone working in a museum is likely to get
thrown off the track by the common name Black-capped.
“I
agree that Black-hooded for nigricapillus is suboptimal given that it is
less hooded than destructus. I don't personally see nigricapillus as
particularly capped (the contrast vs the cheek argument put forward earlier)
but being pragmatic, this name is not going to cause a slew of mis-ID's in the
field or mayhem in collections. Whatever
your views of the cheek needing to contrast or not, nigricapillus does
have black on the cap/crown and it aligns with the scientific name, so I am
fine with Black-capped for nigricapillus. Further, I also think
NACC should have priority on the naming of this one, so I'm not sure that this
vote really matters but Black-capped is my vote for nigricapillus.
“For
destructus I really think either Black-hooded or Choco serves just
fine. I favor and vote for Black-hooded for two reasons:
1) Alignment with Black-capped and with the
original name, Black-headed.
2) Given that at some
point Black-faced Anthrush, F analis is likely going to be split, I think it's
quite likely we'll end up with Amazonian and Central American Antthrush as
common names and those will pair well with Mayan, so perhaps avoiding a
geographic descriptor for destructus helps to keep a bit of name
alignment not just now but also in a future split of Black-faced.”
Additional
comments from Josh Beck (guest vote): “I had assumed that Black-headed would be too
disagreeable.
“Without
having requested a data dump from eBird to get precise numbers, a quick look at
eBird and Macauley and XC shows that, as expected, both daughters are commonly
observed. I would estimate destructus is a bit more commonly observed,
perhaps a 60/40 split in data, give or take. For me this doesn't particularly
impact a vote in this case but wanted to put the info out there in case it
sways others.
“I
personally have no problem with Black-hooded and Black-capped but would prefer
Black-headed and Black-hooded and am not worried about confusion here, so would
vote for Black-hooded and Black-headed. I am actually quite catholic as well
about which of the two carries which name. Black-headed for nigricapillus
if it is viewed as more appropriate, or Black-hooded for nigricapillus if
there is desire to align with Howell & Dyer's recent precedent.”
Comments
from Andrew Spencer (voting for Naka): “I've also really been enjoying the
discussion around these names! I've given myself a couple of days to think
about these, and my initial gut reaction hasn't changed that much: I'm
relatively ambivalent about the proposed names, but I am very against keeping
the old name around (unless it's as compound names for both split species,
which is probably overkill here). The fact that we have to even think about
which one is better to use the old name for is especially a red flag for
me that we should be retiring it if at all possible. If this was an instance
where there was absolutely no acceptable options for one of the split species I
might be more accepting of retaining the pre-split name, but I don't think that
is the case here.
“On
to the various names that people have proposed. I honestly think that the
"capped" look of nigricapillus is, at best, very
subtle. And for the times that it is most apparent, the "cap"
looks very dark brown contrasting with the black elsewhere on the head, rather
than truly "Black-capped". That being said, it would hardly be the
first time a common name draws attention to a subtle plumage feature, and I
prefer "Black-capped" over retaining the old name by many miles.
“’Black-hooded’
is ok for destructus. I'm also completely fine with ‘Choco’, but the
symmetry of using plumage features for the two split species tilts my vote
towards that for this bird as well.
“So,
my official vote for now is to follow the names Nacho originally proposed. If
that ends up not passing though, and we're spit-balling new names, what about
using "Central American Antthrush" for nigricapillus, and
then "Choco Antthrush" for destructus? At the moment
there are no other antthrushes endemic or almost entirely limited to Central
America (unless you consider the Yucatan part of Central America). When
Black-faced Antthrush ends up getting split, the vocal type in Central America
makes it well into Colombia, so contra what Josh says I think the name
"Central American" works better for nigricapillus than it will
for that eventual species.”
Comments
from Naka:
“A) Species limits:
YES, I think we can safely consider F. nigricapillus and F.
destructus two separate biological species based on the evidence provided
by Areta & Benítez Saldívar (2025).
“B) English names:
After giving a careful read to all the comments, I would recommend Black-capped
Antthrush for F. nigricapillus and Choco Antthrush for F.
destructus.
“C) As for the presence
of F. nigricapillus in South America (and therefore within our working
area), I agree with Van, in giving a YES vote, but urge the Colombian National
Committee to decide on this one.”
Comments
from Stiles:
“A: YES. C. “YES to maintaining nigricapillus on SA list, given the
number of cases in other groups in which a Central American or Panama species
just makes it into Colombia, suggesting a lack of ecological barriers at this
point.”
Comments
from Zimmer:
“A. YES. The data from Areta &
Benítez Salvador (2025), as summarized nicely in the Proposal, are both
clear-cut and convincing, particularly as regards the analysis of differences
in loudsong between the two taxa. In
retrospect, I remember being struck by how different destructus sounded
the only times I encountered it in Ecuador, relative to nigricapillus,
which I knew well from Costa Rica and Panama.
Since my only encounters with destructus were from a single trip
in 1993, one in which every other memory and experience of the trip was
overwhelmed by news of Ted Parker’s death just two days into my trip, I had
frankly forgotten all about looking into the vocal differences any further
until I saw this Proposal. C. YES. Cerro Tacarcuna records in Colombia should
be provisionally considered to be nigricapillus until shown otherwise.”
Comments
from Robbins:
“A. YES. This seems straightforward based on the differences in the primary
vocalizations, so yes for recognizing destructus as a species. C. “YES,
pending decision by Colombian list committee.”
Comments
from Claramunt:
“A. YES. Clearcut differences in song, plumage, and morphology indicate these
are fully separated lineages. C. NO. Correct me if I’m wrong, but the Cerro
Tacarcuna record does not have tangible evidence available for verification.
Therefore, we can’t include F. nigricapillus in our list anymore. The
record doesn’t fulfill our criteria for Inclusion. Votes need to be
reconsidered.”
Additional
comments from Remsen:
“C. I change my vote to NO on this one – I think Santiago’s reasoning is the
way we should go. There is no tangible
evidence that the Colombian report pertains to either species; besides not
having a specimen, photo, or sonogram, the report doesn’t even have a written
description that supports the record as pertaining to either species. I have no doubt that the observers had this
one right, and to think that this could be destructus is
biogeographically implausible. But our
criteria do not allow for “probability” to affect the decision.
“Here
is the account from Renjifo et al.:
‘BLACK-HEADED
ANTTHRUSH Formicarius nigricapillus
Solitary
individuals, or with mixed-species flocks, observed following army-ant swarms
above 1,000 m
at our study site. Previously recorded in the Chocó lowlands at Nuquí north
to Bahía
Solano (D. Calderón-F. pers. comm.), the río Jurubidá
on the Pacific coast (Haffer
1967a, Hilty
& Brown 1986) and Serranía de Abibe (D.
Calderón-F. pers. comm.). The closest
locality in
Panama is Nusagandi, western San Blas (Ridgely &
Gwynne 1989). Black-faced
Antthrush F.
analis inhabits the Urabá region and the Atrato Valley, but the contact zone
between the
two species was not previously known. Haffer (1967a) suggested the Alto del
Buey area (1,810 m), in the Serranía del Baudó, as a
possible contact zone. However, one
record of
Black-faced Antthrush on the Panamanian slope of Cerro Tacarcuna at 1,180 m
(Sullivan et
al. 2009), plus an aural record below 800 m at our study site suggests possible
contact in
the foothills of Cerro Tacarcuna, where the species possibly replace each other
elevationally.
Specimen collection will be necessary to test Haffer’s
hypothesis that the two
species may hybridise in a narrow zone within this region.’
Additional
comments from Stiles:
“C. I´m OK to change my vote to NO in accord with Santiago's comment that there
is no confirmed record of nigricapillus for Colombia.”
Comments
from Bonaccorso:
“A) Yes. The data
(acoustic, genetic, and morphometric) support two good biological species.
“C) NO. Visual reports
should not be enough to include nigricapillus in the SACC list. A
photograph or a good recording could be considered as ‘tangible.’"
Additional
comments from Zimmer:
“C. I’ll switch my vote on this to adhere to our criteria in spite of
plausibility.”