Proposal (1048) to South American Classification Committee
Revise
species limits in Habia rubica: A. Treat Middle American H.
rubicoides as a separate species; and B. Also treat Amazonian rhodinolaema group as a separate species.
Note from Remsen: This
is a proposal submitted to NACC (as “Treat
Red-crowned Ant-Tanager Habia rubica as six or seven species“)
that I here modify for SACC. First, is
the text of the original proposal, left largely intact, including the emphasis
on Middle American taxa not directly relevant to SACC; you can skip big chunks
of this. Then, I included comments from
NACC members. Finally, I restructure the
proposal and voting along lines consistent with the NACC results and relevance
to SACC.
Background
The Red-crowned Ant Tanager Habia rubica (Cardinalidae) is a highly polytypic taxon with marked geographical variation; up to 14 subspecies have been recognized, most of which were
described based on variation in the hue and intensity of plumage color
(Hilty, 2011). Its current distribution ranges from
central Mexico to northeastern Argentina and southeastern Brazil and encompasses
regions with very different ecological conditions or separated by recognized
biogeographical barriers (Hilty, 2011).
Ridgway (1902) placed Habia rubica in the tanagers, which
included 21 genera. One of them was Phoenicothraupis
(Cabanis 1850). According to
Ridgway (1902), the genus Phoenicothraupis
(described by Cabanis 1850 with the type Saltator rubicus Vieillot) had three species: Phoenicothraupis rubica
(with five subspecies: P. r. rubicoides, P. r. nelsoni, P. r. vinacea, P. r. affinis, P. r. rosea),
Phoenicothraupis salvini (with five
subspecies), and Phoenicothraupis
fuscicauda (monotypic). Ridgway (1902) described the genus Phoenicothraupis as follows:
Medium-sized
Tanagers superficially resembling the more uniformly colored species of Piranga, but outermost (ninth) primary
shorter than second (instead of decidedly longer than third); adult males with
a scarlet crown-patch and with more or less red on under parts (sometimes confined to the throat); females
and young brown
or olive above,
paler below. Bill as in the more slender-billed species of Piranga
but narrower (width at base scarcely if at all exceeding basal depth), the
gonys relatively shorter, and distinctly, though slightly, convex, and
maxillary tomium without any indication of a tooth-like projection. Nostrils
narrower. Rictal bristles strong, conspicuous, and frontal bristles (over
nostrils) well developed. Wing about three and three-fourths to a little more
than four times as long as tarsus, much rounded (seventh to fourth primaries
longest, ninth shorter than second); primaries exceeding secondaries by much
less than length of tarsus. Tail shorter than wing by much less than length of
tarsus, sometimes nearly as long as wing, more or less rounded, the rectrices
rather broad, with rather loose webs and somewhat pointed tips. Tarsus
decidedly longer than middle toe with claw; outer claw reaching about to or a little beyond
base of middle
claw, the inner
claw falling short of the latter; hind claw shorter
than its digit.
Coloration.—Adult
males reddish brown, reddish gray, or dusky, with bright red throat and crown,
the feathers of the latter
sometimes developed into a more or less obvious
crest; females and young usually brownish above, paler beneath, with or without
a yellowish-buffy or tawny
crown-patch; adult female
sometimes similar to the male,
but duller.
Range.—Southern
Mexico to southern Brazil, Paraguay, Bolivia, and western Ecuador.
Phoenicothraupis was placed in Habia by Hellmayr (1936) and subsequent classifications. The genus Habia was
until recently placed
in the Thraupidae, but we now treat
it in the Cardinalidae (Burns et al. 2014).
The
AOS currently treats
Habia rubica as single
species (AOU 1983,
AOU 1998). Hilty (2020)
recognized 17 subspecies as follows:
·
H. r. rubica: southeastern Brazil (southern Minas Gerais) to eastern Paraguay and
northeastern Argentina
·
H. r. bahiae: tropical
eastern Brazil (Bahia)
·
H. r. rubicoides: southern
Mexico (Puebla and eastern Veracruz)
to northern Nicaragua
·
H. r. holobrunnea: subtropical eastern Mexico (southern Tamaulipas to Veracruz and
northern Oaxaca)
·
H. r. nelsoni: southeastern Mexico (Yucatán Peninsula north of southern Campeche)
·
H. r. alfaroana: northwestern
Costa Rica (Guanacaste Peninsula)
·
H. r. vinacea: Pacific
slope of southwestern Costa Rica (Nicoya
Peninsula) to eastern Panama
·
H. r. affinis: Pacific slope
of southern Mexico (Oaxaca)
·
H. r. rosea: Pacific
slope of southwestern Mexico (Nayarit and Jalisco to Guerrero)
·
H. r. rubra: Trinidad
·
H. r. crissalis: coastal
mountains of northeastern Venezuela (Anzoátegui to Sucre)
·
H. r.
mesopotamia: Venezuela (Río Yuruán region of eastern Bolívar)
·
H. r. coccinea: eastern
base of eastern Andes of north-central Colombia
and western Venezuela
·
H. r. rhodinolaema: southeastern Colombia east of the Andes to northeastern Peru and far northwestern Brazil
·
H. r. peruviana: tropical
eastern Peru to central Bolivia
and adjacent western Brazil
·
H. r. hesterna River (eastward
to Rio Xingu), southward to northern Mato Grosso
·
H. r. perijana: Sierra de
Perijá (Colombia/Venezuela border)
New information:
Three recent studies were published on Habia rubica. One (Lavinia et al. 2015) was a phylogeographic
analysis that suggested that this species originated in South America, where
there are at least two clearly differentiated clades: one in the Atlantic
Forest of Brazil and another in the Amazon basin. However, limited
taxon-sampling precluded detailed
investigation of diversification
within Central America and southern Mexico. The other two studies (Ramirez-Barrera 2018, 2019) provided
new evidence, one using multilocus data and the other, plumage color.
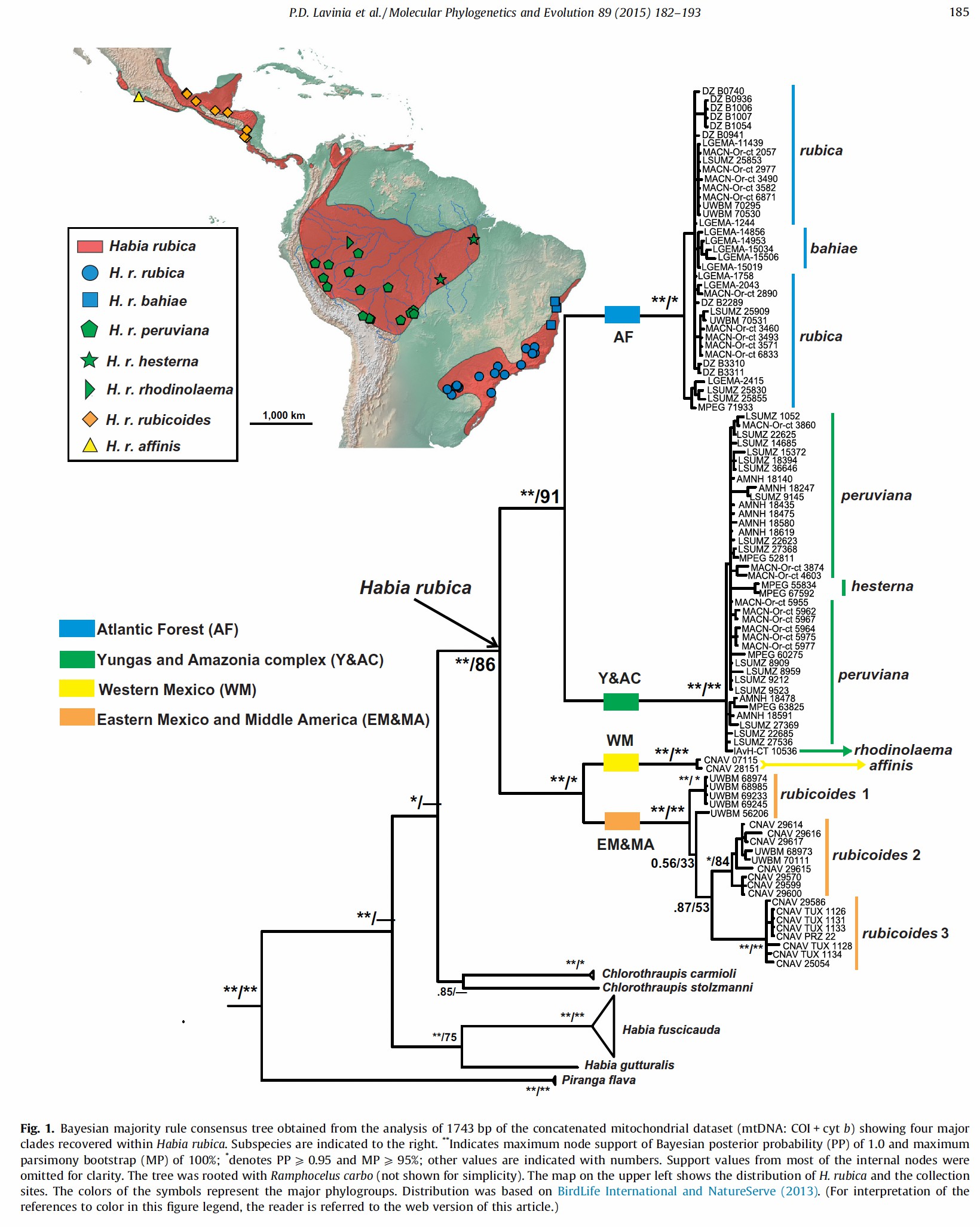
Lavinia et al. (2015) sequenced mtDNA and nDNA from 100
individuals from Mexico to Argentina. Their results are shown below (Bayesian
and maximum parsimony, Fig. 1). Their geographic sampling did not include Panama
and much of western Mexico.
The most important result was the identification
of two lineages in South American that split ca. 3.5 MA and two lineages in Middle America. Their analyses of
vocalizations and plumage coloration found differences between the four main
groups consistent with the molecular data.
Ramirez-Barrera et al. (2018) amplified mitochondrial and
nuclear markers from 125 individuals of H.
rubica covering the species’ distribution, the other three species of Habia (fuscicauda, atrimaxillaris, gutturalis),
and 16 samples from Chlorothraupis (C. olivacea, C. carmioli and C. stolzmanni). They found that H. rubica can
be divided into three main clades: (1) western Mexico, (2) eastern Mexico to Panama, and (3) South
America. Within these
main clades they recognized
seven main phylogroups, as shown in the next figure:
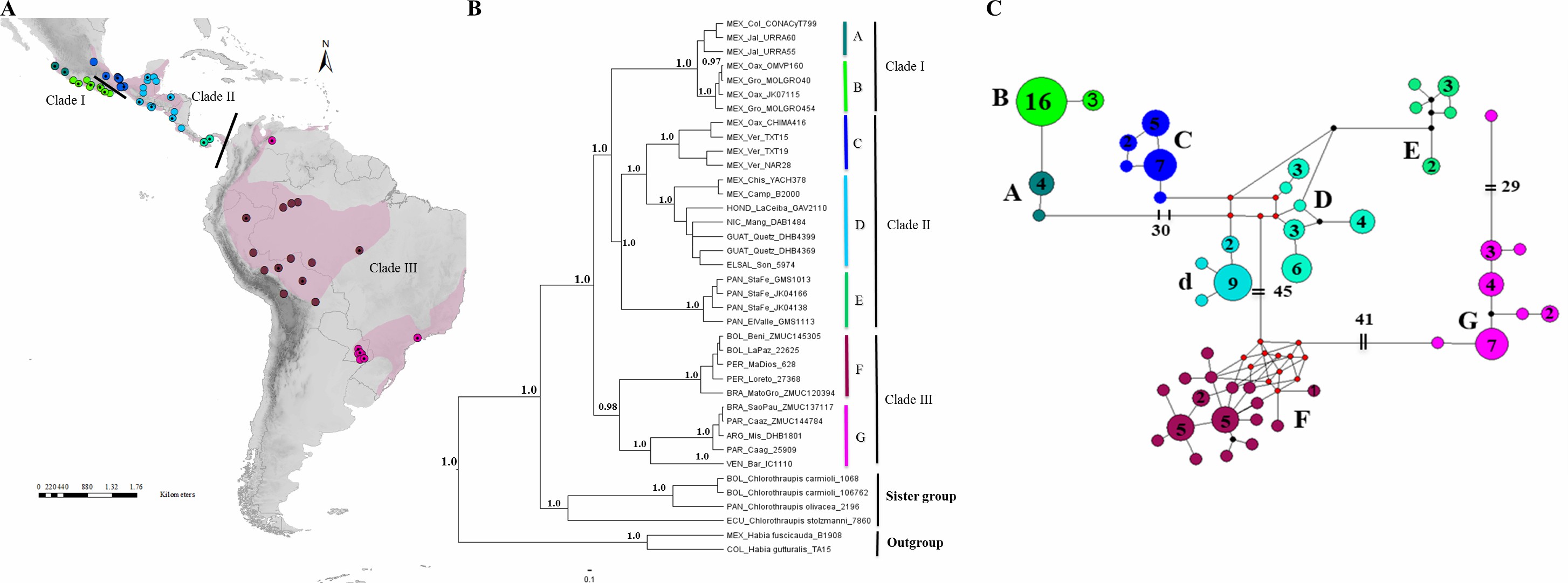
Figure 1. Geographical
distribution, phylogenetic consensus tree, and haplotype network. (A)
Geographical distribution (indicated by pink shading) and sampling points of H. rubica; the mitochondrial DNA
sampling is represented by the color of the dots and the nuclear DNA sampling
is highlighted with a black dot on the dot’s color. ArcGIS (ArcMAP 10.2.2;
Esri, Redlands, CA, USA). (B) Phylogenetic consensus tree representing the relationship among populations of H. rubica, based on Bayesian inference
from a multilocus dataset. Values above branches denote posterior probabilities
(PP). (C) Haplotype network, where the phylogroup ”D'' corresponds to
individuals from the Chiapas-Yucatan peninsula to Costa Rica and “d''
corresponds to individuals from Guatemala and El Salvador
(the numbers inside
of circles indicate
the number of individuals
that shared each haplotype).
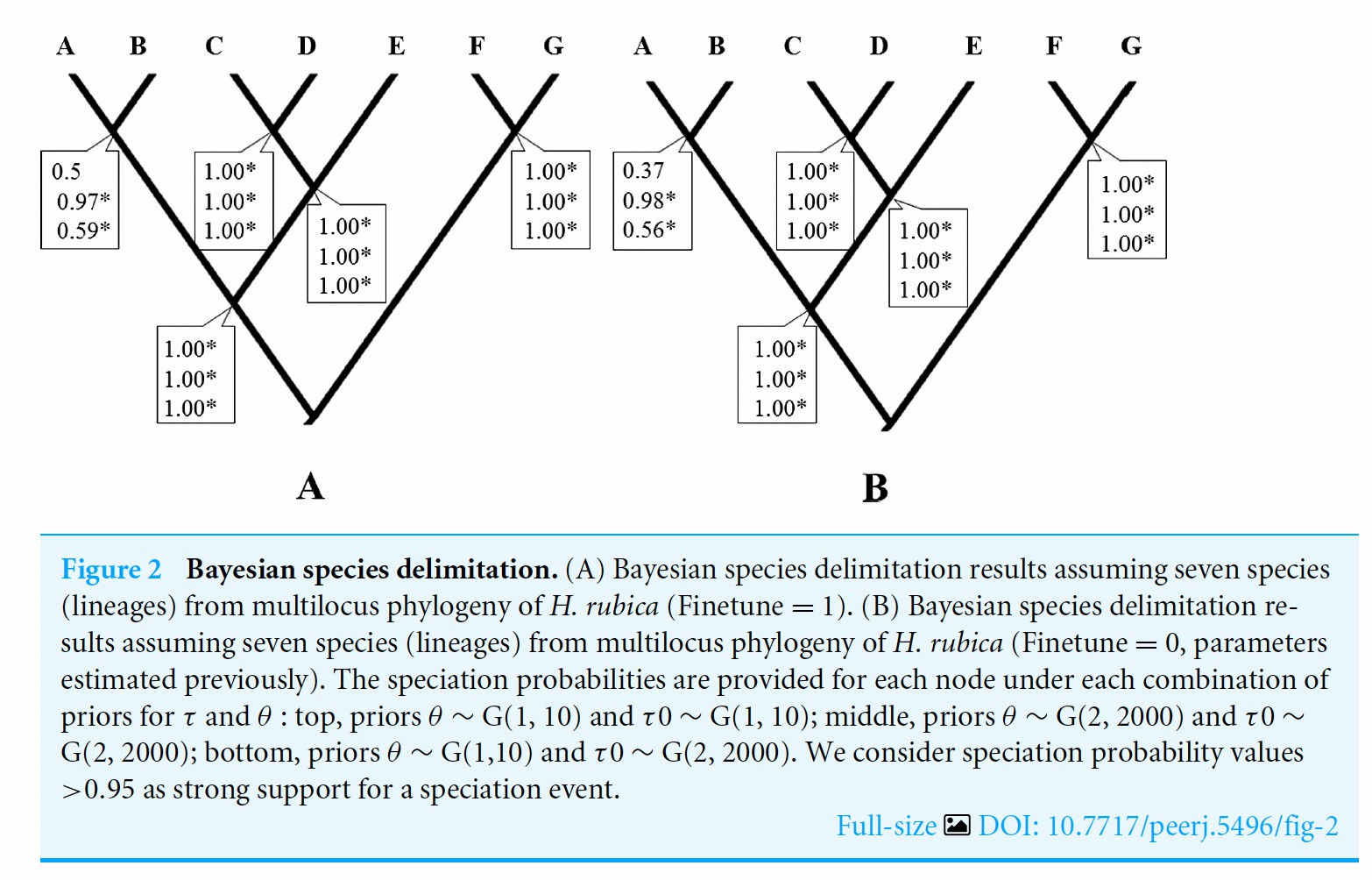
Ramirez-Barrera et al.’s (2018) species
delimitation analysis (BP&P) is shown on the following page; note the
high speciation probabilities (0.97 to 1.0).
![]() Ramírez-Barrera et al. (2019)
analyzed genetic, coloration, and morphometric data from specimens from
collections in Mexico and the United States and used the Multiple Matrix
Regression with Randomization (MMRR) approach to evaluate the influence of geographic
and environmental distances on genetic and phenotypic differentiation at both
the phylogroup and population levels. They found that geographic isolation was
the main factor structuring genetic variation within populations of Habia rubica; this suggests that climate
did not playing a major role in within-species genetic differentiation.
Ramírez-Barrera et al. (2019)
analyzed genetic, coloration, and morphometric data from specimens from
collections in Mexico and the United States and used the Multiple Matrix
Regression with Randomization (MMRR) approach to evaluate the influence of geographic
and environmental distances on genetic and phenotypic differentiation at both
the phylogroup and population levels. They found that geographic isolation was
the main factor structuring genetic variation within populations of Habia rubica; this suggests that climate
did not playing a major role in within-species genetic differentiation.
Recommendation:
We present two options:
(1)
Separate H. rubica into
seven species: This is based
on phylogenetic evidence and some differences on plumage color.
1.
Habia rosea (Nelson, 1898): Pacific coast of western
Mexico (Jalisco, Nayarit,
and Colima; lineage A in
Figure 2)
2.
Habia affinis
(Nelson, 1897): Pacific
coast of southwestern Mexico (Michoacan, Guerrero,
and Oaxaca; lineage B in Figure 2)
3.
Habia holobrunnea (Griscom, 1930): E Mexico from S Tamaulipas S to N Oaxaca (lineage
C in Figure 2
4.
Habia
rubicoides (Lafresnaye, 1844): S Mexico (from Puebla, E
Oaxaca, Tabasco, and Chiapas), Guatemala and Belize S to Honduras,
El Salvador, and Nicaragua (lineage
D in Figure 2).
5. Habia vinacea (Lawrence,
1867): Panama (lineage E in Figure 2)
6. Habia rhodinolaema (Salvin
and Godman, 1883): Amazon basin (lineage F in Figure 2)
7. Habia rubica (Vieillot, 1817): southeastern Brazil,
Argentina, and Paraguay
(lineage G in Figure 2)
(2) As
in Option 1 but treat lineages A and B as the same species (Habia rosea – Habia affinis). This option is to separate Habia rubica into six species based on two main arguments, the
first being the low speciation probability value (<0.95) presented by the
clade comprising the subspecies H. r.
rosea and H. r. affinis in the
BP&P analyses performed with multilocus data (Ramírez-Barrera et al. 2018).
The second argument is the consistency of this grouping through two independent
tests of the same analysis and with adjustment
of specific parameters. At the morphological level, both subspecies are described as a polytypic group characterized by
having paler plumage than the other subspecies of H. rubica (del Hoyo
2020). At the geographical level, the distribution of these two subspecies from
western Mexico is clearly delimited by three large mountain ranges: the Eje
Neovolcánico Transversal, the Sierra Madre del Sur, and the Sierra Madre
Oriental. These geographical formations seem to have great influence on the
genetic structure of the populations, acting as barriers to gene flow, which
has possibly promoted the differentiation between the populations of eastern
and western Mexico.
|
Species |
Proposal 1 |
Species |
Proposal 2 |
|
1 |
Habia rosea (Nelson, 1898) |
1 |
Habia affinis (Nelson, 1897) |
|
2 |
Habia affinis (Nelson, 1897) |
||
|
3 |
Habia holobrunnea Griscom, 1930 |
2 |
Habia holobrunnea Griscom, 1930 |
|
4 |
Habia rubicoides (Lafresnaye, 1844) |
3 |
Habia rubicoides (Lafresnaye, 1844) |
|
5 |
Habia vinacea (Lawrence, 1867) |
4 |
Habia vinacea (Lawrence, 1867) |
|
6 |
Habia rhodinolaema (Salvin and Godman, 1883) |
5 |
Habia rhodinolaema (Salvin and Godman, 1883) |
|
7 |
Habia rubica (Vieillot, 1817) |
6 |
Habia rubica (Vieillot, 1817) |
We recommend Option 1 for the following reasons:
1. The phylogenetic evidence
supporting the differentiation between H.
r. rhodinolaema and H. r. rubica in
South America is consistent across two independent studies (Lavinia et al.
2015, Ramírez-Barrera et al. 2018), which
present broad sampling
and cover most of the distribution
of both phylogroups. In addition, significant differences in traits such as
song and coloration have also been reported (Lavinia et al. 2015), which
support the probable differentiation between the two phylogroups. Furthermore,
there is a geographic correspondence between the distribution ranges of the
identified phylogroups (del Hoyo 2020), which could also indicate that
geographic barriers in South America have influenced their genetic
differentiation.
2. The deep genetic structure
reported between the phylogroups of Central America
and eastern Mexico
(Ramírez-Barrera et al. 2018) shows high support for the phylogenetic
hypothesis obtained with multilocus data. This genetic structure corresponds to
barriers to dispersal and gene flow such as the Talamanca Mountain Range in
Costa Rica, the Motagua-Polochic-Jocotán fault system, and the Isthmus of
Tehuantepec in southern Mexico, which could be promoting the reported genetic
isolation and genetic differentiation. Analyses of differences between these
groups for traits
such as song and coloration could not be tested in detail due to
the low sampling available; however, a clear difference is reported between
these phylogroups and the phylogroups distributed in South America (Lavinia et
al. 2015).
3. The five phylogroups distributed from central Mexico to South America show evidence of genetic and phenotypic differentiation,
as well as geographic correspondence in their distributions. We consider this
evidence sufficient to identify at least five clearly differentiated species.
This would allow us to better explain the relatively weak patterns of variation
among the subspecies described for this geographic range of H. rubica.
4. The phylogroups distributed
along the western slope of Mexico also show robust molecular differentiation
supported by a phylogenetic hypothesis based on multilocus data that shows a
profound divergence between those distributed in the north and south of this
region (Ramírez- Barrera et al. 2018). This evidence is supported by very
complete sampling, which covers the entire distributional range
of H. rubica and
leaves no room for doubt
about the genetic
structure of the species in
this region. In addition, there is evidence of geographic correspondence with
barriers such as the Transversal Neovolcanic Belt, the Sierra Madre del Sur,
and the Sierra Madre Oriental, which limit the distribution of populations and
have kept them isolated for long periods of time, which has favored the deep
genetic differentiation (Ramírez- Barrera et al. 2018). There is no evidence of
song differentiation as in the previous cases; however, both phylogroups are
recognized as a polytypic group whose coloration is markedly paler than that reported
in the rest of the species (del Hoyo 2020), which could add evidence
on the degree of differentiation that these groups present.
5. Habia rubica is
widely described as a polytypic species with a very wide distribution; however,
it has also been described as a species in which “differences between the
racial groups are not always clear-cut or pronounced” (del Hoyo 2020). “Several
of the numerous races are weakly differentiated and seem barely worth
recognizing” (del Hoyo 2020). This apparently weak phenotypic differentiation,
coupled with the complicated field identification of the different subspecies
(especially those with sympatric distribution), makes it necessary to consider
all those genetic, geographical, and phenotypic elements (e.g., pink coloration
of phylogroups from western
Mexico) as sufficient arguments to be able to recognize these
groups as independent species.
English names:
If the proposal passes, a follow-up proposal
on English names is needed.
We should also coordinate with SACC on this proposal.
Literature Cited:
Burns, K. J., A. J.
Shultz, P. O. Title, N. A. Mason, F. Keith Barker, J. Klicka, S. M. Lanyon, and
I. J. Lovette. 2014. Phylogenetics and diversification of tanagers (Passeriformes: Thraupidae), the largest radiation of Neotropical
songbirds. Molecular Phylogenetic and Evolution 75: 41-77.
Hilty, S. L.
2011. Family Thraupidae (Tanagers). Handbook of the birds
of the world. Tanagers to New World blackbirds, vol. 16.
Barcelona: Lynx Editions, 46 329.
Lavinia, P. D.,
P. Escalante, N. C. García, A. S. Barreira, N. Trujillo-Arias, P. L. Tubaro, K. Naoki
, C. Y. Miyaki, F. R. Santos,
and D. A. Lijtmaer. 2015. Continental-scale analysis reveals deep
diversification within the polytypic Red-crowned Ant Tanager (Habia rubica, Cardinalidae). Molecular
Phylogenetics and Evolution 89:182 193.
DOI 10.1016/j.ympev.2015.04.018
Ramírez-Barrera, S. M.,
B. E. Hernández-Baños, J. P. Jaramillo-Correa, and J. Klicka. 2018. Deep
divergence of Red-crowned Ant Tanager
(Habia rubica: Cardinalidae), a multilocus phylogenetic analysis with emphasis in
Mesoamerica. PeerJ. 1-19 DOI: 10.7717/peerj.5496
Ramírez-Barrera, S. M.,
J. A. Velasco, T. Orozco-Téllez, A. M. Vázquez-López and B. E. Hernández-Baños. 2019. What drives genetic and phenotypic divergence in the Red-crowned Ant Tanager (Habia rubica, Aves: Cardinalidae), a
polytypic species? Ecology and Evolution. DOI: 10.1002/ece3.5742
Ridgway, R. 1902. The birds of North and Middle America:
a descriptive catalogue of the higher groups, genera, species, and
subspecies of birds known to occur in North America, from the Arctic lands
to the Isthmus of Panama,
the West Indies
and other islands
of the Caribbean Sea, and
the Galapagos Archipelago. Bulletin of the United States National Museum No.
50, Part 2.
Blanca E. Hernández-Baños & Sandra M. Ramírez-Barrera, May 2025
Here
are the comments from NACC members (names removed to protect anonymity as per
NACC policy):
Voter 1: “YES to A. I find the combination of very old
(mid-Pliocene!) genetic lineages and qualitative differences in song and
plumage to be indicative of multiple species in the group. However, until
someone quantifies the differences in song and plumage, I am only comfortable
with recognizing three (and at most four) species in this group: the Atlantic
Forest, Amazonian, and Middle American lineages. There seem to be some
consistent differences with the two West Mexican lineages, and if other
committee members move towards recognizing that clade as a species, I am open
to the idea.
“So, for now, I will vote to
recognize as species the 3 clades listed above. The Lavinia et al. (2015) study
cited in the proposal does have some data on song and plumage, but they had
data mostly just for the Amazonian and Atlantic Forest clades, but they showed
that the latter has a darker / more saturated red plumage and a distinctly
slower song. The part that concerns me is that they mention that there was more
plumage variation within each clade than between. This agrees with my
experience with the species, which like many Cardinalids shows considerable
variation in the carotenoid-based colors, even within one flock. A thorough
study of plumage variation would be helpful, but even with those caveats in
mind, there are consistent differences between the clades in my experience. The
Atlantic Forest birds, in addition to being redder, are a distinctly intense
and evenly colored red that is quite different than that of the Amazonian and
Mesoamerican birds. The females are also distinctly brown (vs olive). There
also seem to be some qualitative structural differences with the Atlantic
Forest birds, especially in the head and bill shape. I was unfamiliar with the
song of this group, but in listening to recordings, they do have very widely
spaced notes, typically repeated, sound ‘clipped’, and are quite different than
the other clades. The calls also seem shorter. In contrast, the Amazonian birds
are somewhat paler red, more often have gray on the flanks, have a more
contrasting red throat, have clear whistled songs that are run together and
rise to a kind of crescendo at the end, and have longer harsher calls. The
Mesoamerican birds look to me more like the Amazonian group, but there is so
much plumage variation that I’m not sure if I can pick out a consistent difference
versus the Amazonian birds (a bit concerning). The Mesoamerican birds do have
distinctly rising notes that are given in pairs, at least in the north. The
southern Central American birds tend to have a kind of bouncy staccato song,
which likely warrants investigation. The ones in West Mexico are clearly pale
and small-billed, but again, there is much variation. The songs are more
slurred, too. These do seem to be allopatric from the populations in eastern
Mexico and are a distinct lineage on the tree.
“We will likely need another
proposal on English names if this passes, but I’ll just toss some ideas out
there. First, I don’t think that we should further modify “Red-crowned”, as
that will create unnecessarily long names. eBird/Clements has subspecies group
names, which are a good starting point, but do not perfectly match the clades
if we adopt fewer than five species. Given the brighter red plumage of nominate
H. rubica, I suggest following eBird/Clements’ and adopt Red Ant-Tanger
for this clade. For the other two, I suggest geographic or plumage-based
names. eBird/Clements uses Scarlet-throated Ant-Tanager for rubra
of the Amazon, which fits for the bird given its brighter throat, but could I
suppose cause a perceived association with Red-throated Ant-Tanager. Amazonian
Ant-Tanager could also work, but this species would also include the perijanus
of the Perijá mountain range (outside the Amazon). Regardless, both are SACC
issues. For our birds, eBird/Clements uses “Northern” for rubicoides of
Panama to east Mexico and “West Mexican” for the west Mexican clades. I suggest
Mesoamerican or Middle American Ant-Tanager for the combined group.”
Voter 2: “YES to A. As with other committee members, I
am most comfortable now with recognizing three species: Atlantic Forest,
Amazonia, and Middle America. The Lavinia et al. (2015) study had only one
sampling locality for western Mexico (n = 2), and that clade was also not
included in the vocal analysis. Furthermore, I am concerned by the lack of
study of any potential contact zone in Middle America; the only mention of a
contact zone there is the statement in the paper that “the possibility of a
contact zone in southern Oaxaca and Chiapas remains to be explored and
therefore the presence of gene flow cannot be fully discarded.” Until such a
study is done, I think it’s best to wait on splitting western Mexico
populations from those in eastern Mexico and Middle America.
Voter 3: “(A) YES; split the species; (B) Three species. I’ve
enjoyed reading the series of detailed papers on this complex; there are
definitely multiple species involved. There is good evidence for 5 or 6 species
under many concepts, such as the phylogenetic species concepts. However, since
we adhere to the biological concept, I think we need more evidence regarding
the proposed Mexico and Central American species, in particular vocal data. As
others have noted, to date we have song, plumage, and genetic data to justify a
three-way split (Atlantic Forest, Yungas & Amazonia, and Mexico/Middle
America). Thus, at this point, I would be in favor of voting for a 3-way
option. As a side note, one of my MS students has an unpublished MS thesis
(Scott 2019) with a UCE phylogeny that includes one sample each of these three,
and it shows the same set of relationships as the studies discussed in this
proposal. Levels of relative divergence is similar to divergence among
currently recognized species of other cardinals/grosbeaks.
Voter
4: “YES to a split. The
proposal and the papers it is based on make it clear that at least three BSC
species are involved. I also consider that the evidence favors treating the two
West Mexican taxa as a separate species (affinis) rather than as a
subspecies of rubicoides, based on the genetics, morphology, and song.
Song.---I downloaded and compared
songs of the two groups in sonogram composites, and it seems pretty clear that
the notes of affinis songs are more modulated and more vertical, while
the notes of rubicoides are more prolonged and horizontal, and often
with multiple note types per strophe rather than a single note type or a tiny
grace note as is typical of affinis. It also seems that affinis may
not sing that often, with many more recordings of calls than song, while there
are plenty of songs of rubicoides. Calls of these two groups are less
tractable, with multiple call types represented, some perhaps misidentified
(?), and not differing obviously in quick comparisons. (If anyone wants to see
the sonogram composites I’d be happy to share).
Plumage.---Not necessarily a species characteristic, but I
was quite impressed with the pallid plumage of birds I saw in Jalisco last
year, which seemed very unlike those I’ve seen elsewhere.
Genetics.---The two phylogenies presented in the proposal
show a divergence between the west Mexican and east Mexican/Central American
taxa more or less on a par with the other clades.
Distribution.---It’s not clear from either the range maps in
Howell and Webb, or eBird records, that there even is a zone of overlap between
the affinis and rubicoides groups. There seems to be a small
break in Oaxaca between the northern Oaxaca rubicoides group and
southern Oaxaca affinis group.
“So, I’m voting for four
species (first choice) and failing that, three species (second choice).
Voter 5: “YES. As other committee members have already
commented, I am only comfortable accepting a 3-species split, recognizing
Middle American, Amazonian, and Atlantic Forest birds as separate, although I
too would be open to also recognizing the West Mexican subspecies as separate
if the rest of the committee feels that is appropriate.”
Voter 6: “YES. I agree with the other committee
members that there is good evidence for a 3-way split of Middle American,
Amazonian, and Atlantic Forest birds. There may very well be additional splits,
but I’d like to see more analyses of phenotypic variation and putative contact
zones among the Middle American taxa. BP&P analyses certainly have value
but tend to oversplit lineages compared to more conventional species
delimitation approaches.”
Explanation
of SACC voting procedure from Remsen: As a consequence of these comments and votes,
NACC voted to recognize three species, as follows:
1. Habia rubicoides
(extralimital to SACC): includes all Middle American taxa listed in the
proposal, from Mexico to Panama.
2. Habia rhodinolaema: Amazonia etc. (includes peruviana and hesterna by genetic sampling and perijana, rubra, crissalis, mesopotamia, and coccinea by phenotype; note that rhodinolaema was not genetically sampled)
3. Habia rubica: Atlantic
forest (including bahiae)
Note
that the NACC proposal really didn’t get into the details of the South American
taxa – the focus was on Middle America.
So, let’s try this as a voting structure, with two YES/NO choices.
A. TWO SPECIES. YES is
to treat Middle American H. rubicoides as a separate species from all
South American taxa, i.e. treat the rhodinolaema and rubica groups as a single species, at least for
now. NO is for continuing the status quo, i.e., all one species. A NO vote on this automatically translates to
a NO on B (I think).
B. THREE SPECIES. YES is to treat all three
groups as separate species, as per NACC voting results. NO is for continuing the status quo, i.e.,
all one species, or for more than two species in South America (an option not
considered in the NACC proposal).
Note
from Remsen on English names: A separate proposal will be needed on English
names if either part of the taxonomic proposal passes, but meanwhile note
discussion in NACC comments.
Voting Chart: https://www.museum.lsu.edu/~Remsen/SACCPropChart1044+.htm
Comments
from Bonaccorso:
“NO. I think the level of discussion for South American-Panamanian forms in the
current proposal is not sufficient to make an informed choice. The three
proposed species are clearly divergent in mitochondrial DNA, but the level of
nuclear divergence is difficult to tell because the concatenated mtDNA-nuclear
tree could be driven by mitochondrial divergence. Plumage differences, however,
are consistent with the subspecies level.”
Comments
from Stiles:
“YES to A and B. For A, this definitely shifts the burden of proof onto those
recommending the status quo. B is a well-substantiated spilt based genetic and
vocal evidence as well as more subtle differences in plumage. The split also
makes biogeographic sense because the two populations are rather widely
allopatric, following an Amazonia vs. eastern Brazil separation seen in many
other groups.”
Comments
from Areta:
“Well, as routinely happens, the lack of a paper
focusing on species-limits issues integrating data and a proliferation of
papers lacking complete taxon sampling creates a situation in which some things
appear to be obvious, some appear to be likely correct, and some other issues
go undetected or at least are not given the foreground place that they deserve.
I find it highly intriguing that none of the papers, nor the proposal, have
cited Parkes (1969), which is a highly relevant paper discussing important specimens
(e.g., the distribution of rhodinolaema
and coccinea, and the birds west of
the Andes in Colombia) and characterising two new subspecies from Venezuela (crissalis and mesopotamia).”
“Clade III in Ramírez-Barrera et al. 2018 is the South American clade, which is
deeply diverged from Clades I and II in Mesoamerica that should be the focus of
our deliberations. The deep split between these taxa was also recovered by
Lavinia et al. (2015). Thus, it is clear that there are two very deeply
diverged main lineages, one in South America and the other one in Mesoamerica.
mtDNA and concatenated mt+nucDNA are concordant.
“Lavinia et al. (2015) made the following taxonomic
recommendations:
‘ 4.5.
Taxonomic implications
We found H.
rubica to be monophyletic within our dataset with high support in spite of
the deep divergences among its main lineages. The Crested Ant Tanager (H.
cristata), a species that has not yet been included in any molecular
studies and that based on morphological and behavioral evidence seems to be
closely related to H. rubica (Willis, 1972),
should be included in future analyses to confirm this result. In addition, and
as evidenced by Klicka et
al. (2007) and Barker et al. (2015), H. rubica appeared more closely
related to species of the genus Chlorothraupis than to its congeneric
species, making the genus Habia paraphyletic as currently defined. Since
further analyses including H. cristata are needed to definitely
establish the phylogenetic affinities among the species of these two genera, we
are anyway keeping the genus name Habia for the new species suggested
below.
“Based on
consistent genetic, morphological and behavioral evidence, we believe that
splitting H. rubica into three species is the most reasonable and
conservative alternative for the moment. One of the species should be the
lineage present in the Atlantic Forest, which should keep the name H. rubica.
Despite the fact that our genetic analyses do not distinguish between the
subspecies rubica and bahiae from the Atlantic Forest, we believe
that further morphological and behavioral objective analyses (like the ones
performed here among main lineages) are needed to fully address their validity.
Therefore, we propose that this species should keep the subspecies rubica
and bahiae for the moment. Although we lack representatives of some
races distributed north of the Amazon River, we recommend that all South
American subspecies other than the ones from the Atlantic Forest are included
in a new species called H. rubra (given its taxonomic priority). Until a
more comprehensive sampling of the subspecies from northern South America
allows further genotypic and phenotypic analyses of this group, H. rubra
would include the subspecies rubra, peruviana, rhodinolaema,
hesterna, perijana, coccinea, crissalis, and mesopotamia.
Finally, we suggest considering the Mexican and Middle American lineage a
single species named H. rubicoides. This species would temporally
comprise the subspecies rubicoides and affinis, as treated by Navarro-Sigüenza and Peterson (2004), and alfaroana and vinacea,
until further sampling in western Mexico and southern Middle America allows a
deeper analysis of these subspecies."
“The deepest splits in Clade III are group F (which we can call
the rhodinolaema complex) and group G
(which we can call the rubica AND rubra complexes).
“The rhodinolaema complex: taxon rhodinolaema was apparently sampled by
Lavinia et al. (2015) but not by Ramírez-Barrera et al. (2018) (or so I
understand; it is tough to check all the samples for ssp. assignation, which
would have ideally been done by the authors; maybe it was sampled only for
mtDNA?), so its recognition would rest on the sampling of rhodinolaema, hesterna,
and peruviana (i.e., all its
constituent taxa).
“The rubica
complex: a tight grouping of AF birds, coupled to vocal distinctions
indicate that it deserves to be ranked as a species different from rhodinolaema.
This would include ssp. rubica and bahiae, both genetically sampled.
The rubra
complex: this group was not recognised as such by any of the published
papers or proposals, but I think that it can be reasonable to propose its
existence. One sample from the eastern base of the Andes of Venezuela (coccinea) is eye-catching, as it is
recovered as part of III-G, but it could as well have been placed on its own
"lettered" clade given the depth of the divergence and the
biogeographically implausible case of a species having a disjunct
distributional pattern in the E Andes of Venezuela and Colombia and in the
Atlantic Forest (see map in Ramírez-Barrera et al. 2019, which makes this more
evident). This Atlantic Forest/northern South America connection is in need of more attention. Interestingly, the subspecies rubra from Trinidad and crissalis, from the NE mountains of
Venezuela (Paria and Turimiquire; type from "Mirasol (3,000 feet), about
15 km. S. of Cumanacoa, Sucre, Venezuela") have very similar "double-
or triple-ticking" calls to coccinea
and perijana (https://search.macaulaylibrary.org/catalog?taxonCode=rcatan1&mediaType=audio&sort=rating_rank_desc&view=list®ionCode=VE),
and very unlike the harsh long rasp of Atlantic Forest birds or the dry, short,
staccato calls of the rhodinolaema
complex. Taxon rubra, the second
oldest name for Habia rubica sensu
lato, from Trinidad is also missing genetic data. Populations west of the Andes
in Colombia seem to also belong to this group and lack a name (if one is
needed; see Parkes 1969). Finally, the taxon mesopotamia from SE Venezuela (E Bolivar) also lacks samples (both,
vocal and genetic) and it was not even mapped by Lavinia et al. 2015 or
Ramírez-Barrera et al. 2018, 2019). So, we lack sampling of key subspecies
level entities that would presumably occur within the Clade III.
“I think it is troublesome to place the northern
Andes/Paria-Turimiquire/E Bolivar taxa in the same species with the Atlantic
Forest birds, when the admittedly meager genetic and vocal data indicate that
they are deeply diverged and vocally distinctive (as in the proposal). I
likewise think it is problematic to mix the rubra
and rhodinolaema complexes under an
enlarged H. rubra as in Lavinia et
al. 2015 (i.e., include every South American taxon here, except for H. rubica rubica and H. rubica bahiae).
“It seems that there are AT LEAST 2 species in clade G rubica (with rubica and bahiae) and rubra (including, it seems, rubra,
crissalis, coccinea, and perijana,
and one could stretch this to include mesopotamia).
This is a classic problem: whether we should follow our gut feeling, experience,
and sense of likelihood, or whether we should wait for solid data. I tend to
follow the latter approach, because this is how solid knowledge is built. So
far, I have proposed a different taxonomic hypothesis to those presented in the
proposal or by Lavinia et al. 2015, but to me this hypothesis has not been
rigorously tested. This group is still begging for a taxonomically minded
genetic work, and for more sound recordings.
“After writing all this, I´ve found that eBird has a "rubra" group, that includes what I
have here termed rhodinolaema and rubra complexes. These two are better
treated separately, based on the single genetic sample from Barinas of coccinea in Ramírez-Barrera et al. (2018)
and on the vocal differences of perijana,
coccinea, crissalis, rubra (and
maybe also the unrecorded, at least on XC and ML, mesopotamia) from the rhodinolaema
complex.
“What to do? There are hundreds of sound recordings that are crying to be
analysed. In a rapid assessment, it seems that they can help diagnose at least
3 main vocal (call) groups in Clade III which agree with genetic data (but many
ssp. need more vocal samples, some remain unrecorded, and some are lacking
genetic data). So, I think that
Clade III might better be treated as at least 3 species: rubica (with ssp. rubica
and bahiae), rhodinolaema (with ssp. rhodinolaema,
hesterna, and peruviana), and rubra
(with ssp. rubra, coccinea, birds W of the Andes in
Colombia?, and perijana, and perhaps
even mesopotamia, although this one
might as well be something different, and the composition of this third species
remains speculative).
Other scenarios are likely as well, one species could be Andean (coccinea and perijana), one limited to NE Venezuela (rubra) and another one to E Venezuela (and Brazil? Guyana?; mesopotamia). I think that the 3-species
solution might eventually be correct, but do we have enough data to decide?
What to do? Move forward piecemeal or wait for an integrative approach? I look
forward to the thoughts of others, knowing that I am usually on the "wait
for thorough data" team.
“Disclaimer: it seems to me that there are numerous situations here that should
be properly sorted in a rigorous taxonomic work. I approached this with an
inquisitive mind, and I think that I could grasp part of the complexity of the
situation. However, revising type (and other) specimens, assessing the
distributions of taxa, properly sampling for genetic data, and studying the
vocalizations should be done with greater attention to detail than what I could
afford to vote here. Yet, I think that I have uncovered a novel pattern that
should be tested. Maybe I erred here and there, but I feel that the overall
picture is accurate, and so here it is to be subjected to scrutiny and
criticism.”
Comments
from Lane:
“NO for now. As Nacho points out, it seems the original proposal did a
disservice in its layout of the clades and the proper use of nomenclatural
priority. I have not had the luxury of time and access to the LSU collection to
review and think about this properly, and so I will take the coward’s way out
and wait until I can give this case more study before I vote for change.”
Comments
from Naka:
“In light of the info provided I agree with a novel treatment within Habia.
“A. YES, I agree with
splitting the South American clades from the Middle American ones, which have
notable genetic and some morphological differences.
“B YES, I agree with
considering the two large clades in SA as distinct species, something that we
have done in the past within the Brazilian Committee. However, prior to making
this split, we need to properly define which name applies for the Amazonian
taxa. I am yet unconvinced that the name Habia rhodinolaema (Salvin and
Godman, 1883) applies for the entire clade.
“According to Lavinia
et al (2015) cited in this proposal: "we recommend that all South American
subspecies other than the ones from the Atlantic Forest are included in a new species
called H. rubra (given its taxonomic priority).", which is the
approach we took in Brazil a few years ago. If we are to use rhodinolaema,
we need to make sure this is the correct approach. But that seems to be a
technical issue that can be solved once the decision is made.”
Comments
from Claramunt:
“A reluctant YES to both A and B. The tree groups are clearly delineated
genetically but the phenotypic evidence is blurrier in the papers and in the
proposal. What are the phenotypic traits that would diagnose the three clades?”
Additional
comments from Stiles:
“B. still YES (the nomenclature can be settled later, as per Nacho).”
Comments
from Robbins:
“YES. When this proposal was first posted I spent some time and concluded that
I needed to revisit, which I have just done. Germane to our committee, Nacho
has delineated issues that need to be addressed. Nonetheless, based primarily
on the genetic data, I think it is reasonable at this point in time to
recognize at least three species: Middle American (likely more than one
species) and at least two species in South America. So, I vote YES to both A
& B.”
Comments
from Remsen:
“NO to A and B. I have no doubt that
additional data will continue to show that more than one species is involved,
but there are just too many unknowns.
“This
complex contains 17 recognized subspecies taxa, and the initial proposal
recommended dividing these into 7 species.
Vocal data have not been formally analyzed. Plumage data are essentially irrelevant to
species rank unless populations are in direct contact (at which point they may
serve as proxies for gene flow) or unless there is evidence that they are used
in mate choice. As noted by Elisa, nDNA or genomic data are absent except for a
UCE data-set involving a couple of taxa in an unpublished thesis (see Burns’
NACC comments), but even if they were available, I don’t think they are
relevant for assigning species rank unless taxa are in direct contact or unless
they are completely beyond anything known for taxa ranked as conspecific. The 3.5 mya divergence time is well within
the range of numerous sedentary tropical taxa treated at the subspecies rank,
so cannot be used as a reason for assigning species rank. I look forward to the day when decisions on
species-level taxonomy based solely on gene trees using neutral loci are no
longer considered valid.
“I
strongly support Nacho’s philosophy on this.
It feels sometimes as if we are rushing to make some splits when we know
more than one species is involved, or our gut tells us that additional data
will support preliminary qualitative information. What’s the rush? Unless there is an urgent conservation issue,
I see no reason not to wait for more rigorous, complete analyses on such a
widespread complex for which vocal and genetic data are or soon will be
available.”
“If
I were to undertake a study of this complex, the first thing I would do is
assemble a list of the 17 available names and their type localities. Then, I would assess whether these 17 names
refer to phenotypes that can be diagnosed by plumage; I think one could get
away with a semi-qualitative analysis in most cases. Habia rubica specimens are common in
collections. Borderline situations might
require quantitative analysis. This
would give you a list a valid taxa that should be
sampled. Then, I would assess whether any of these taxa are clearly parapatric;
if so, then an absence of intermediate phenotypes at the contact zones is
sufficient, in my opinion, for assigning species rank; this would put
burden-of-proof on treating them as conspecific. Then, I would see what is
available in terms of genetic and samples of presumed homologous vocalizations
that can be definitely assigned to a taxon based on proximity to type
localities. Lavinia et al. and Ramírez-Barrera et al. provide great assistance
in all this. Then, I would proceed with quantitative analyses of those data,
with careful attention to diverse geographic sampling especially proximity to
contact zones if they exist.”
Comments
from Zimmer:
“A & B) “YES and YES. This is an
incredibly complex and case, given the number of named taxa involved, the
geographic span (Mexico to NE Argentina & SE Brazil), the gaps in taxon
sampling, and the lack of any quantified vocal analyses. Add to that, the lack of analyzed topotypical
material (genetic, vocal, phenotypic) means that we don’t even know how many
named taxa are even valid, leading to additional nomenclatural uncertainty as
to how to recognize clades identified by the genetic studies. In spite of all of that, I think it is
undeniable that the status quo of 1 species, is incorrect. I think the genetic data make clear that
there are at least 3 species here (Don’t forget that the original NACC Proposal
was for a choice between recognizing 7 species or 6, and everyone seemed to
vote “YES” but then proposed recognizing some different number of species.),
and the proposed breakdown of Middle/Central American, Atlantic Forest, and
everything else, while almost certainly oversimplified in my view, at least
represents an advance from the single species treatment that ‘moves the ball
down the field’. Further work will
likely result in a more granular subdivision of the Middle American and the
Andean/Amazonian/Guianan/northern Venezuelan clades, but I think what we
already know of genetic, vocal and phenotypic differences is sufficient to
split both the Middle American and Atlantic Forest taxa from one another and
from everything else. I have a fair
amount of field experience with Middle American taxa and with South American
taxa from outside of Brazil, but setting that aside, and looking at Part B of
this Proposal solely from a Brazil-centric perspective, it makes total sense to
me that nominate rubica (with ssp. bahiae), would be treated as a
distinct species from all of the taxa found in Amazonian Brazil (hesperna, peruviana & rhodinolaema). Atlantic Forest birds (rubica & bahiae)
share 2 types of songs, one, a distinctively phrased song with a slow cadence,
which, in my experience, is mostly a “dawn song”; and the other, a faster,
run-together sequence that is more often given during the day, while family
groups are foraging together (often as nuclear members of
mixed-species-flocks), and which are typically interspersed with the calls. Neither the dawn song nor the other song is
close to songs from any of the populations in Amazonia, nor have I heard
anything similar from elsewhere (outside of Brazil) in South America, or, from
anywhere in Middle/Central America.
Also, calls of Atlantic Forest birds are audibly different from those of
Amazonian birds, and the calls, which in Habia are given more frequently
than the songs, are probably especially important in maintaining group cohesion
when moving through the forest understory.
As for plumage differences, I would argue that the differences between
Atlantic Forest birds and the most geographically proximate Amazonian taxon (hesperna), particularly as they relate to the female
plumage (see photos below), are at least as great as those between Middle/Central
American populations of rubica and sympatric H. fuscicauda. In sum, the current evidence, while painting
a very incomplete picture, and while inadequate in many respects, at least
allows us to advance our treatment of the Habia rubica complex beyond
the current status quo, and in doing so, should serve as a springboard for the
more integrated and thorough analysis that this complex requires.
“The
2 photos below are of specimens from the LACM collection:
Top
photo: Lateral view from L to R: 2 female hesperna
from R Tapajós, Pará, Brazil; 1 female nominate rubica from São Paulo,
Brazil; and 1 female bahiae from Alagoas, Brazil.

Bottom
photo: ventral view, from L to R: 2 females and 1 male of hesperna
from R Tapajós region, Pará, Brazil; 1 female and 1 male of nominate rubica
from São Paulo, Brazil; and 1 male and 1 female of bahiae from Alagoas,
Brazil.

Comments
from Niels Krabbe (guest voter):
“A:
YES Split C American from South American forms.
“B: YES Split the Atlantic rubica with bahiae from remaining
South American forms.
“Both
trees agree in the split between Central American and South American birds.
Within South America, the vocal differences mentioned by Kevin and the dark
bill and underparts shown on the photos convince me that a split of
southeastern birds (rubica with bahiae) is warranted. As Nacho
suggests in his detailed comments, there will likely be at least one more split
to come (rubra group from rhodinolaema group), but until then, rubra
of Trinidad has priority for the remaining South American forms.
“I
find it rather unsatisfactory that Scott et al. (2024) apparently did not
upload their sequences to GenBank (also SACC proposal 1007).”
Comments
from Areta:
“"NO. We need a full taxonomic work integrating genetic data (available
for the most part, with some key holes), biogeographic thinking, vocalizations,
nomenclatural matters, and plumage. Until then, we have suggestions of
different possible treatments that may, or may not, pass the test of time. At
present, as I argued, I am convinced that there are more species in South
America than originally envisioned in the proposal, and I laid out the
possibilities there. If someone is willing to undertake the comparative work to
refine species limits in a coherent framework and publish a scientific work,
I´d be happy to reconsider. Otherwise, our treatment would be the result of a
blend of data and serendipity, instead of the product of a rigorous
taxonomically-minded work produced to clarify species limits. Thus, with a
heavy heart, I vote NO to A and B."
Additional
comments from Niels Krabbe: “Following Nacho's (and Elisa's) argumentation, and
awaiting Chesser's new sequences to be uploaded to GenBank (Scott et al. 2024),
I change my vote to NO for both A and B.”