Proposal (1052) to South American Classification Committee
Revise species limits
in South American Bubo owls: (A) Maintain broadly defined B.
virginianus; (B) Treat magellanicus as a separate species from B.
virginianus; (C) Treat nigrescens as a separate species from North
American B. virginianus, irrespective of the former’s status with
respect to magellanicus; (D) Treat nigrescens a subspecies of B.
magellanicus; (E) Also treat B. nacurutu as a separate species from B.
virginianus, restricted to North and Middle America.
Background: Proposal 328 was a first attempt
laid out before SACC to change the taxonomy of Great Horned Owl (Bubo
virginianus) in splitting off the austral form B. (v.) magellanicus
as its own species. The complex had first been reviewed by Traylor (1958), who
laid out the distribution and known taxonomy of the South American populations,
with a northern Andean subspecies (nigrescens, type locality: “Cechce”
<sic: Ceche, Chimborazo; Paynter 1993>, Ecuador), a southern Andean and
Patagonian subspecies (magellanicus, type locality: Tierra del Fuego), a
lowland savanna subspecies (nacurutu, type locality: Paraguay; including
scotinus from Caicara, Río Orinoco, Venezuela, and elutus from
Lorica, Bolívar, Colombia), and a caatinga form (deserti from Salitres,
near Joazeiro, Bahia, Brazil). As Mark Robbins has laid out the situation of
the taxonomy of the group in the previous proposal, I won’t run over it much
further here. However, largely due to a set of confounding recordings by Ted
Parker from the northern Andes of Peru (Cerro Cruz Blanca, Piura) associated
with a specimen at LSU (LSUMZ 97577) that has been identified as “Bubo
virginianus nigrescens?” (see images), the SACC rejected the proposal at
the time. These recordings seemed to represent a population of Bubo in
the Andes of northern Peru that shared both “northern” and austral song types.


Since
then, multiple authorities have supported the split, including Clements
checklist (Clements et al 2025)—and by extension, eBird—, IOC checklist (Gill
et al. 2025). This split within the American Bubo has been proposed by
various authors due to the distinctive song and smaller size of the austral B.
(v.) magellanicus (e.g., Koenig et al. 1999, Jaramillo 2003, Pearman and
Areta 2020) in comparison to the geographically nearby lowland form B. v. nacurutu,
particularly because of no habitat or elevational overlap between the two
despite very close proximity in Argentina (see comments by Jaramillo in Prop
328).
The first publication on the genetics of the complex (Ostrow et al. 2023)
showed a deep branch between B. (v.) magellanicus (including samples
from as far north as the Andes of Peru) and the remainder of the B.
virginianus complex, though not without some messiness. So, it is time for
SACC to re-evaluate the situation.
Analysis: At the time that Mark
drew up Proposal 328, we had been stymied by the absence of better voice and
genetic specimen sampling along the northern Andes. Since that time, two papers
have reviewed both the vocalizations of the American Bubo (López-Lanús
2015) and the genetics (Ostrow et al. 2023). Both have supported the split of B.
magellanicus from B. virginianus but have not suggested any further
changes to the taxonomy within the latter. In addition, new recordings, from
Ecuador and Colombia, have been added to Macaulay Library (see below). I will
discuss the voice and the molecular studies separately below.
Voice: So, to assess is each of
these new papers. López-Lanús (2015) did an exhaustive analysis of the
vocalizations of all populations of the Bubo virginianus complex from
Alaska and Canada south to Tierra del Fuego using online sound archives
(Macaulay Library and Xeno-canto). He concluded that there were five song types
represented: (1) the widespread North American voice (hereafter called “virginianus”),
(2) the Patagonian magellanicus, (3) savana nacurutu, (4) northern
Andean nigrescens, and (5) also considered the Parker recordings
mentioned above to represent an undescribed sixth voice type (which he called
“Ñécu” and which he proposed to be an undescribed taxon). Interestingly, the virginianus
voice is very strongly conserved throughout North America and is diagnosably
different in pattern (sex for sex) from both nacurutu and nigrescens.
I should point out, that in López-Lanús’ study, the least-represented
population in the sound collections he checked was nigrescens with N=4.
Since that time, additional recordings have been archived, particularly in
Macaulay Library, and these are key to the situation with nigrescens and
magellanicus. Importantly, these recordings include several duets, which
apparently are the circumstance when nigrescens gives the puttering
series that Parker recorded in Piura and that is so similar to magellanicus.
These recordings of the puttering duet span the distribution of nigrescens
from the Eastern Andes of Colombia to Piura, Peru:
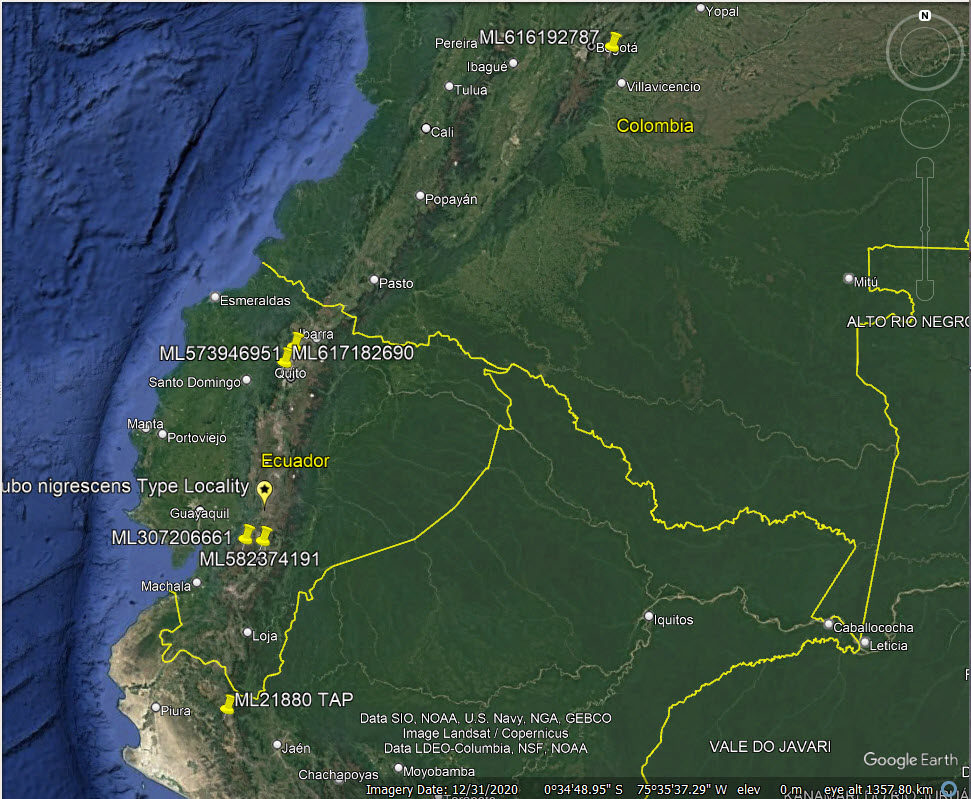
ML cuts of B. v. nigrescens giving
puttering notes like B. magellanicus: ML616192787 (Cundinamarca,
Colombia), ML307206661 (Azuay, Ecuador), ML582374191 (Cañar, Ecuador), ML573946951, ML617182690 (Pichincha, Ecuador), ML21879, ML21880, ML21890 (Piura, Peru). See map
figure.
It
is this song type that López-Lanús (2015) considered his “new” voice “Ñécu!”
Apparently, it is actually a representative song type over the entire
distribution of B. v. nigrescens, and this may require a new view on the
relationships of that taxon.
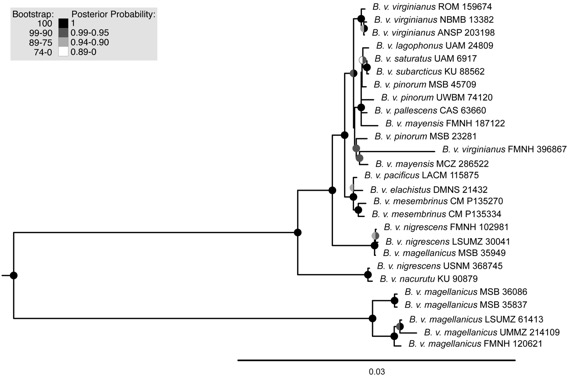
Genetic analysis: Ostrow et al.
(2023) used both nuclear UCEs and mtDNA over a large area of both the North and
South American distribution of the Bubo virginianus complex, although I
should note that North American samples greatly outnumbered South American;
some specimens were strictly sampled from toepads (including 2 nigrescens
and 2 magellanicus). The sample sizes of the South American taxa were
reported as: nigrescens 3, nacurutu 1, magellanicus 6.
However, I must point out that one of the “nigrescens” (a toepad sample)
was from La Guajira, Colombia, and therefore was actually a nacurutu.
This translates to an actual sample of nigrescens 2, nacurutu 2,
and magellanicus 6. In Ostrow et al. (2023), this misidentification
resulted in nigrescens being paraphyletic in their Figure 2 (all
samples) which is a maximum-likelihood UCE tree, with the La Guajira sample
being sister to the “single” nacurutu sample, although this now makes
sense as both samples were nacurutu.
I will point out that no southern nacurutu (which are closer to
the type locality) were sampled, so it would be useful to know how much
structure there is within the taxon, particularly between northern and southern
populations.

On
the “all samples” tree, the nacurutu branch comes out within the North
American virginianus group among samples that are largely from the
southwestern USA and Middle America. Sister to the North American/nacurutu
group is nigrescens, with magellanicus sister to this entire
clade. In the “fresh tissues only” tree of Figure 2, the North American clade
is sister to nacurutu (so: the two are monophyletic with respect to one
another) with nigrescens sister to them and magellanicus sister
to that entire clade. Figure 3 of Ostrow et al. is a maximum-likelihood tree
using mtDNA. This tree has the North American virginianus as a
monophyletic clade with its sister being nigrescens and a specimen of magellanicus
(from Lima, Peru). Sister to these clades is nacurutu (including the
misidentified “nigrescens”), and finally, the branch with the remainder
of magellanicus. Ostrow et al. (2023) concluded that there was still
gene flow between nigrescens and magellanicus (both Andean taxa)
in Peru, and also (some gene flow between one of the nigrescens (Cauca,
Colombia) and North American birds (!).
These genetic results have me
scratching my head a bit: the trees presented in the main paper of Ostrow et al.
(2023) seem not to have concordant branching among the North American birds, nacurutu,
and nigrescens, but all or the bulk of magellanicus is always
sister to that entire clade. But the positions of nacurutu and nigrescens
with respect to the North American birds switch depending on the tree, and a
Peruvian magellanicus is on the nigrescens branch on the mtDNA
maximum-likelihood tree. I can’t imagine that nigrescens and North
American birds are currently experiencing gene flow across the Darien (nor have
they in a long time!).
Recommendation: The publications since
Proposal 328 have made a bit of a hash of the distribution and taxonomy of the
complex, in part because of errors in assigning names to populations, in part
due to missing data that have since become available. The voice and phylogenetic
placement of B. magellanicus with respect to the rest of the B.
virginianus complex seems to make a strong argument that it should be
recognized as a separate species. However, there appears to be molecular
evidence of continuing gene flow between magellanicus and nigrescens
in Peru AND the vocal repertoire of nigrescens actually contains the
puttering component that makes magellanicus so distinctive as to result
in its split by so many authorities! So, if the gene flow between the two taxa
is real, why aren’t they on a single branch apart from the remainder of the
complex? Could it be a sampling artifact?. Finally, even though nacurutu
and nigrescens are both later branches off the main B. virginianus
tree with respect to B. magellanicus, each clade has distinctive voices
compared to the virginianus voice, and neither is actively experiencing
gene flow with populations of the North American virginianus group (the
separation between nigrescens and the nearest virginianus is
between the Central Andes of Colombia and Costa Rica, and similarly between nacurutu
in the lowlands of northern Antioquia, Colombia, and Costa Rica). My gut
feeling is that we should divide up the B. virginianus complex into four
species: B. virginianus (all taxa south to Panama), B. nacurutu
(lowlands of South America, including the populations named scotinus, elutus,
and deserti, the validity of which remain to be reviewed), B.
nigrescens (Andes of Colombia to Piura, Peru), and B. magellanicus.
Either this or continue to maintain the full group as one species, as it seems
that nigrescens and magellanicus share the “distinctive”
puttering that has been the main character used to separate the latter from the
remaining complex, and that Ostrow et al. found evidence of continuing gene
flow between the two in Peru. Comprehension of the molecular results of Ostrow
et al is not exactly in my wheelhouse, so I will leave it to those on the
committee who can address them better and how best to vote. So, these are the
options I see:
A.
Maintain Bubo virginianus with all of the South American populations
within it until their relationships are better understood (this seems the least
helpful, I recommend NO).
B.
Separate B. magellanicus from the remainder of B. virginianus
(this seems the favored stance by most other authors and lists, but leaves some
mess swept under the rug, I recommend YES).
C.
Separate nigrescens from North American B. virginianus,
irrespective of the former’s status with respect to magellanicus (vocal
and molecular datasets support this. I recommend YES).
D.
Consider nigrescens a subspecies of B. magellanicus (there is
evidence of some genetic flow between them, and vocally the two share the
puttering vocalization, but it seems to be given under different circumstances
within each. We don’t have much information on what sort of contact zone there
is between the two in Peru. Given that Ostrow et al. do not have any trees
showing nigrescens to be monophyletic with respect to magellanicus,
however, I think this may not be the right move. I weakly suggest NO).
E.
Also separate B. nacurutu from B. virginianus, now restricted to
North and Middle America (given the voice distinctions, especially compared to
the surprising conservativeness within North American populations, I am
inclined to recommend YES).
Literature
Cited
Clements, J. F., P. C.
Rasmussen, T. S. Schulenberg, M. J. Iliff, T. A. Fredericks, J. A. Gerbracht,
D. Lepage, A. Spencer, S. M. Billerman, B. L. Sullivan, M. Smith, and C. L.
Wood. 2024. The eBird/Clements checklist of Birds of the World: v2024.
Downloaded from https://www.birds.cornell.edu/clementschecklist/download/
Gill F, D Donsker &
P Rasmussen (Eds). 2025. IOC World Bird List (v15.1).
Jaramillo, A. 2003.
Birds of Chile. Princeton Univ. Press, Princeton, New Jersey, USA
König, C., F. Weick,
and J-H. Becking. 1999. Owls. A guide to the owls of the world. Pica Press,
Sussex, England.
López-Lanús, B. 2015.
Análisis domparativo de las vocalizaciones de distintos taxa del género Bubo
en América. Hornero 30:69-88.
Paynter, R. A. 1993.
Ornithological Gazetteer of Ecuador, second edition. Museum of Comparative
Zoology, Harvard University, Cambridge, Massachusetts, USA.
Ostrow, E. N., L. H. DeCicco, and R. G. Moyle. 2023.
Range-wide phylogenomics of the Great Horned Owl (Bubo virginianus)
reveals deep north-south divergence in northern Peru. PeerJ 11:e15787
http://doi.org/10.7717/peerj.15787
Pearman, M. and J. I.
Areta. 2020. Birds of Argentina. Princeton Univ. Press, Princeton, New Jersey,
USA
Roesler, I. (2024).
Lesser Horned Owl (Bubo magellanicus), version 1.1. In Birds of the
World (S. M. Billerman and F. Medrano, Editors). Cornell Lab of Ornithology,
Ithaca, NY, USA. https://doi.org/10.2173/bow.grhowl2.01.1
Schulenberg, T.S., D.
F. Stotz, D. F. Lane, J. P. O'Neill, and T. A. Parker, III. 2007. Birds of
Peru. Princeton Univ. Press, Princeton, New Jersey, USA.
Traylor, M. A. 1958.
Variation in South American Great Horned Owls. Auk 75:143-149.
Dan Lane, May 2025
___________________________________________________________________________________________________
Vote tracking chart:
https://www.museum.lsu.edu/~Remsen/SACCPropChart1044-.htm
Comments
from Stiles:
“A. NO. Clearly at least two species are well defined genetically, vocally and
phenotypically. B. YES: the easiest choice; magellanicus is clearly
separate from all the rest. C [now E]. YES to also splitting off nacurutu and
nigrescens as good species. In going over the distributions of all of
these taxa in several recent guides and other treatments, I was surprised at
the wide distribution of nacurutu, which extends from the entire
Caribbean region of NE Colombia through NC Colombia into NW to NE Venezuela,
and also extends through the entire region of the Llanos in both countries (if
nothing else, this form was severely under-sampled in the genetic studies). It
is nearly everywhere a lowland species (ca. 0-600m elevations, although it might
reach higher in extreme NE Venezuela). On the other hand, nigrescens is
entirely montane in its distribution in S Colombia in the Eastern and Central
Andes (and the Western Andes as well?) at elevations of 2500-4000m, south in
the Andes through Ecuador to NE Perú (and Bolivia?). This species is absent
from Venezuela, and there might be some overlap with the range of nacurutu in
Colombia but the two occupy widely different elevations (and given their
different ecologies, vocalizations and genetics, gene flow between them would
seem highly unlikely in the recent past).”
Comments
from Krabbe:
“My votes are:
A: NO
B:YES (split magellanicus from virginianus)
C: YES (split nigrescens from virginianus)
D: NO
E: YES (split nacurutu from virginianus).
“After
listening carefully through the vocal material, I must agree with Dan that the
Piura recordings (ML21879, ML21880, ML21890) are typical of nigrescens
in both hoots and the context of the puttering series. Recordings from
Cajamarca (XC139660) and Lima (ML54603211, XC215522) are like recordings of magellanicus
from Chile and Argentina.
“Besides
the Piura recordings, I thus concur with López-Lanús (2015) in that there are 4
vocal groups: virginianus, magellanicus, nigrescens and nacurutu.
As voice appears to be similar throughout the ranges of each group, it makes
sense to rank all four as biological species, which is also in general
agreement with the phylogenetic trees. Despite the mitochondrial tree
suggesting some gene flow between magellanicus and nigrescens,
the fresh tissue UCE tree places all four in separate branches.”
Comments from Areta: “The conflictive nigrescens (USNM 368745) from Guajira,
Colombia, that falls as sister to nacurutu
(KU 90879) in both the UCE and mtDNA trees in Ostrow et al. (2023) can be
explained away because this bird IS nacurutu
and not nigrescens (based on
geography, altitude, and habitat). Once this is accepted, then the only oddball
seems to be the placement of a magellanicus
sample from ‘Peru, Lima’ (MSB 35949; where exactly from?) as part of the nigrescens clade in the mtDNA tree,
while this sample is well embedded within the magellanicus clade in the UCE dataset. Whether this is true
mitonuclear discordance or there is some other explanation, I don´t know, but
at any rate, the UCE tree solidly places this bird in the magellanicus clade, which would seem to make the most sense
biogeographically (we also don´t know how this specimen looks).
“The differences in vocalizations between virginianus and nacurutu
are constant but I´d say not dramatic, and the same applies to vocalizations of
nigrescens and magellanicus. When taken in conjunction, the patterns of geographic
replacement/parapatry, the differences in plumage, phylogenetic relationships,
and vocalizations as described by López-Lanús (2015) and further clarified by
Dan, tip the scale towards the recognition of four species-level taxa. Much
remains to be done to clarify the extent (if any) of gene flow between the taxa
indicated in Ostrow et al. (2023), and whether past hybridization has played
any role in the vocal features found in nigrescens.
Its jumping phylogenetic position depending on the markers and samples
analysed, and the vocal features shared with magellanicus are intriguing, and this looks like an interesting
research question integrating fieldwork and genomics.
“A:
NO - It seems untenable to have a single species
“B:
YES (split magellanicus from virginianus)
“C:
YES (split nigrescens from virginianus)
“D:
NO - It is not such an easy call, but the phylogenetic trees have never
recovered nigrescens as more closely
related to magellanicus
“E:
YES (split nacurutu from virginianus)”
Comments
from Naka:
“I agree that genetics look messy, but I generally agree with the novel
proposal, as follows:
“A: NO, it's time to
deal with this species.
“B:YES (split magellanicus
from virginianus), easy.
“C: YES (split nigrescens
from virginianus)
“D: NO
“E: YES (split nacurutu
from virginianus), for now. I wonder if further splitting will be
necessary from tropical lowland South America.”
Comments
from Robbins:
“
“A. NO, clearly more
than one species is involved based on vocalizations and genetic data.
“B. YES for recognizing
magellanicus as a species based on voice and genetic data. Yes, the contact zone between it and nigrescens
as well as potentially magellanicus with southern nacurutu need
to be examined (in the development of the project, I pointed out to Ostrow that
several key samples should be incorporated in the study that ultimately
weren’t).
“C. YES for recognizing
nigrescens as a species despite sampling issues as mentioned above.
“D. NO, see comments
above.
“E. YES. I’m more on
the fence regarding this recommendation. Despite mislabeling (I pointed out to
Ostrow that La Guajira, Colombia sample was mis-labeled prior to publication),
much more genetic sampling of nacurutu (see my comments under B) is
needed throughout its range. Nonetheless, based on available vocal data it
appears nacurutu’s voice is consistently distinct from populations north
of South America. So, I support recognition of nacurutu as a species.”
Comments
from Stiles:
“A. NO. B.YES; C.YES; D. NO; E. YES.”
Comments
from Remsen:
“The vocal data are convincing to me that the best taxonomic assessment of this
complex is to treat them as at least three species. I am seriously concerned with the nagging
problems pointed out by Dan with respect to species rank for nigrescens
and I am tempted to vote against species rank for nigrescens until these
details are worked out. On the other
hand, if better sampling does suggest free gene flow between them, then we can
repeal the decision to treat them as separate species. The deciding factor to vote for separate
species rank is the point made by Dan in terms of the homogeneity in virginianus
vocalizations despite remarkable geographic variation in plumage and habitat,
so by a yardstick comparison, consistent vocal differences despite some shared
motifs argues for species rank. Therefore,
my votes conform to those of all voters so far: A. NO. B.YES; C.YES; D. NO; E.
YES.”
Additional
comments from Stiles:
“D. NO to considering nigrescens as a subspecies of magellanicus.
Data on genetics and ecology now available favor considering nigrescens
as a separate species.”
Comments
from Zimmer:
“
“A. NO. Evidence for recognizing at least two species
within this complex is overwhelming.
“B. YES. As Gary says, this is an easy choice, based
upon genetic, phenetic and vocal differences.
“C. YES. The sharp break in vocal differences, along
with the molecular data supports this.
“D. NO. This one is the toughest call, given that the
two taxa share the distinctive “puttering” call, and that there is at least
some evidence for some gene flow, but, ultimately, I am persuaded that these
two are different beasts, by voice, plumage and ecology.
“E. YES. As noted by others, the vocal distinctions
between nacurutu and North/Central American virginianus are not
particularly impressive, but they are consistent, and when compared to the
conserved nature of vocalizations of virginianus throughout North &
Central America, I think this is enough to justify recognition as separate
species.”
Comments
from Claramunt:
“A. NO. Redundant
option.
“B. YES. This is
well-supported by vocal and genetic data.
“C. NO. Given the
modest differentiation in song and the ambiguous genetic data, I think it’s
better to keep nigrescens in the virginianus complex.
“D. NO. The genetic
data clearly show that nigrescens is closer to the main virginianus complex,
not to magellanicus. There may be some signal of ancestral gene flow
between nigrescens and magellanicus but seems minimal, if not
entirely artifactual. The sharing of “puttering” notes may be ancestral to the
group and not indicative of relationships.
“E. NO. There is not
much evidence that nacurutu has separated from virginianus genetically.
Plumage and song differences are subtle.”