Proposal (1054) to South American Classification Committee
Revise species limits
in Rhynchocyclus olivaceus: A. Treat R. aequinoctialis as a
separate species; B. Treat R. guianensis as a separate species; C. Treat
R. cryptus as a separate species.
Effect
on SACC:
This would split widespread Rhynchocyclus olivaceus into as many as four
species, including newly described Rhynchocyclus cryptus.
Background: Our current Note is
as follows:
73a. Boesman (2016) proposed that Rhynchocyclus
olivaceus be treated as two species based on vocal differences, and this
was followed by del Hoyo & Collar (2016).
Simões et al. (2022) found that Rhynchocyclus olivaceus as
currently defined is paraphyletic with respect to R. fulvipectus, and
also proposed that Amazonian populations of R. olivaceus consists of
four species: R. olivaceus of the Atlantic Forest, R. guianensis
of the Guianas and eastern Amazonia, R. aequinoctialis of e.
Panama, northwestern Colombia, and northwestern Amazonia, and newly described R.
cryptus of southwestern Amazonia. SACC proposal badly needed.
Birds
of the World/Clements has instituted a 2-way split: https://birdsoftheworld.org/bow/species/olifla3/cur/introduction?login#sys
New
information:
Simões et al. (2022) densely sampled R. olivaceus and found some fascinating
remarkable results, including strong vocal differences among several of the
groups and sympatry between two of the main vocal groups. This is a classic example of speciation that
would be difficult to detect without genetic and bioacoustic techniques, with
parallels to the amazing case of Turdus sanchezorum, as well as specimen
labels that include habitat type.
Genetics: Simões et al. (2022)
sampled 83 specimens from five of the recognized subspecies of R. olivaceus
(bardus of e. Panama, nw. Colombia, aequinoctialis of w. Amazonia,
guianensis of the Guianan Shield, sordidus of e. Amazonian Brazil
S of Amazon, and nominate olivaceus of the Atlantic forest region of
Brazil. They did not have samples of
four others, all from n. Colombia and n. Venezuela): jelambianus (ne.
Venezuela), tamborensis (nw. Santander), flavus (n. Colombia and
nw. Venezuela), and mirus (Atrato Valley). They sequenced 3 mitochondrial and 2 nuclear
genes.
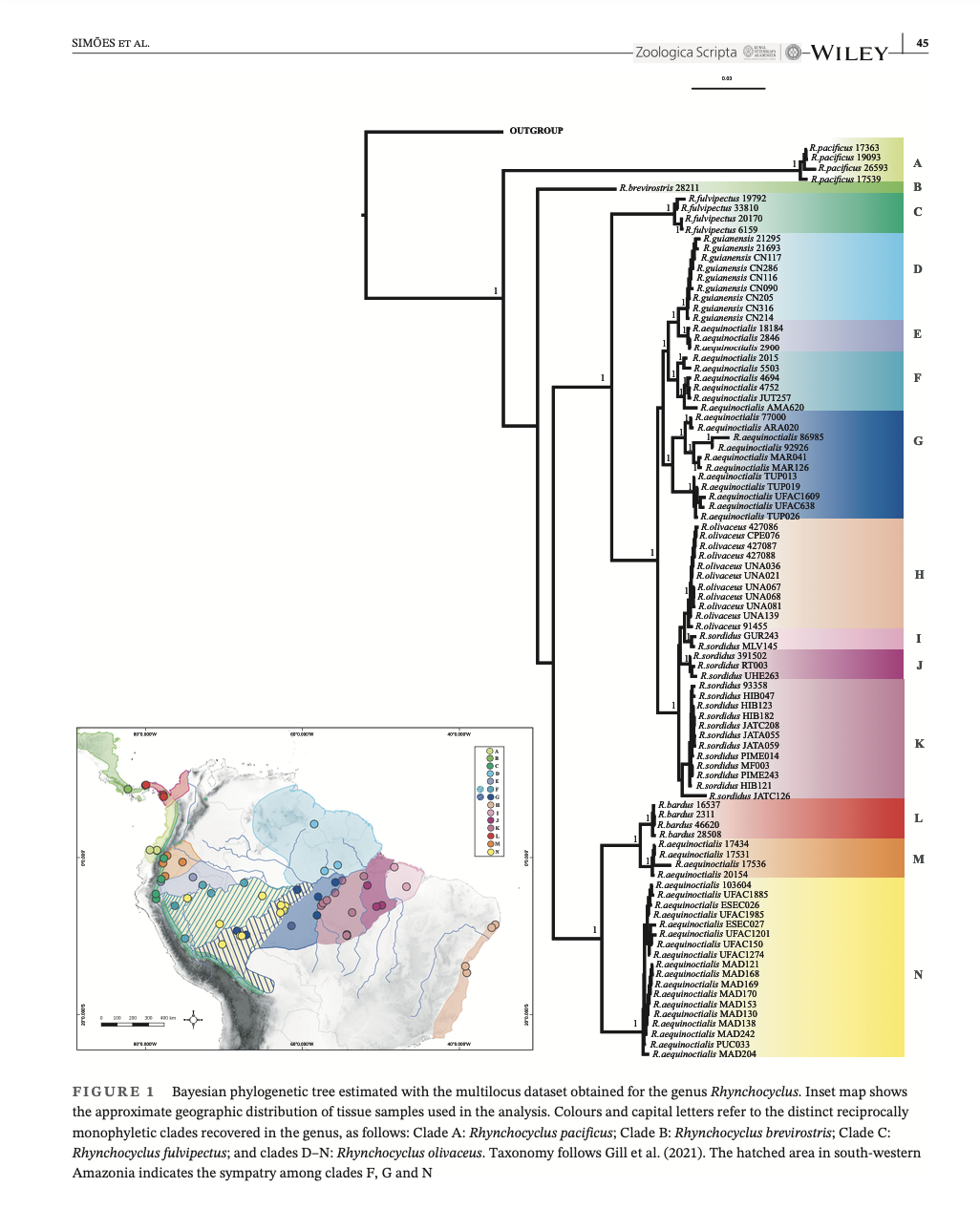
Their
tree (below) showed that R. olivaceus separates into two broad clades,
one of which is sister to R. fulvipectus. One clade consists of aequinoctialis and
bardus, but aequinoctialis is not monophyletic within that
clade. The other clade consists of
nominate olivaceus, guianensis, sordidus, and a different
set of aequinoctialis populations.
(You will need good resolution of this graphic to understand what
follows, so if this screen shot doesn’t accomplish that, then refer to the
original paper – see link in Literature Cited.)
A
preliminary point to note is that, as noted by Simões et al. (2022), their tree
conflicts with the results of Harvey et al.’s (2020) genomic analysis, which
showed that pacificus is not sister to all the others but sister to brevirostris,
as expected on phenotypic grounds because these two were considered conspecific
until recently. This raises a warning
for me in terms of interpretations of the topology of the Simões et al. tree
because of differences in genetic sampling (DNA sequence data for 5 markers vs.
genomic data with 2K+ genomic regions).
With a focus on an entirely different taxonomic level, Harvey et al.
(2020) had only one sample of R. olivaceus (which was sister to R.
fulvipectus), and so no other conflicts can be detected. On the other hand, also note that with the
exception of aequinoctialis, which appears in at least 5 clades, the DNA
sequence data are otherwise generally consistent with subspecies boundaries.
Morphology: Simões
et al. (2022)examined 113 study skins from four of the five subspecies from
which they had tissue samples (missing bardus). They used Smithe (1975)
to characterize color in 8 plumage regions. They took standard morphometric measurements
from the 52 specimens for which sequence data were obtained.
They
were unable to find any diagnostic plumage characters for any of the five
subspecies analyzed, although guianensis were generally brighter yellow
below than the other clades. According
to Cory & Hellmayr (1927), the subspecies can be separated by minor
differences, and although not mentioned directly by Simões et al., their
sampling covered the regions used by Cory & Hellmayr in describing the
differences among the taxa. (I wish that
this had been discussed in more detail with respect to each subspecies, if only
in supplemental material.)
In
terms of morphometrics, none of the lineages was diagnosable, but nominate olivaceus
stood out in PCA spaced as being larger overall than any of the others.
Vocalizations: Simões
et al. (2022) examined 84 song recordings, distributed among all the subspecies
except mirus (including recordings by Dan, Mark, and Gary among current
SACC voters as well as former voting members Bret Whitney, Fernando Pacheco:
see SI https://onlinelibrary.wiley.com/action/downloadSupplement?doi=10.1111%2Fzsc.12519&file=zsc12519-sup-0006-Supinfo.pdf). Because of concerns for homology in vocalizations,
analyses were restricted to what were interpreted as loudsongs, although a few
distinctive call types were also mentioned.
They wrote:
“Unlike
many other suboscine passerines, Rhynchocyclus are usually quiet most of
the time and representativeness of sound recordings in databases is an
important bottle- neck for robust quantitative vocal analyses. Nonetheless, we
evaluated at least one type of song of all species of Rhynchocyclus and
most of described subspecies and clades recovered in R. olivaceus,
except R. o. mirus and clade E birds, which were unavailable
They
found distinct, diagnosable differences for the currently recognized species R.
pacificus, R. brevirostris, and R. fulvipectus. It is great to see these differences
quantified and described in terms of those quantitative data.
Within
broadly defined R. olivaceus, their general results are as follows:
Within
the remaining clades (D–N) and taxa making part of the polytypic and
paraphyletic R. olivaceus, two highly variable loudsong and call types
could be identified, each corresponding to reciprocally monophyletic groups.
One of these vocal groups includes clades D, F, G, H, I, J and K, whereas the
other clusters clades L–N, plus subspecies R. o. tamborensis and R.
o. flavus, which were not sampled genetically (Figures 3 and 4).
In
other words, referring to the figure above, the two main genetic lineages shown
in the tree had notable differences in songs and calls, which they referred to
as Vocal Groups 1 and 2. These
correspond to the two main groups found by Boesman (2016). Below is a sonogram of the loudsong of guianensis:
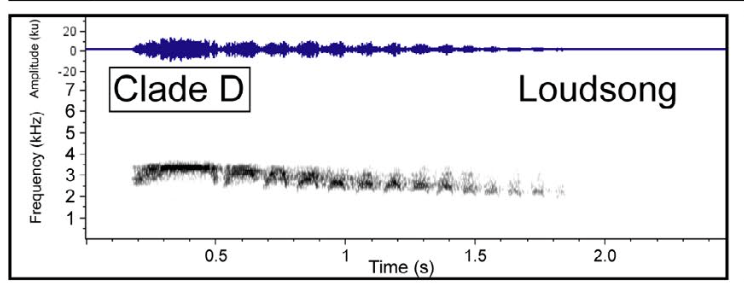
And
below is the sonogram for nominate olivaceus. Note both of these are in the same general
lineage (D-L):
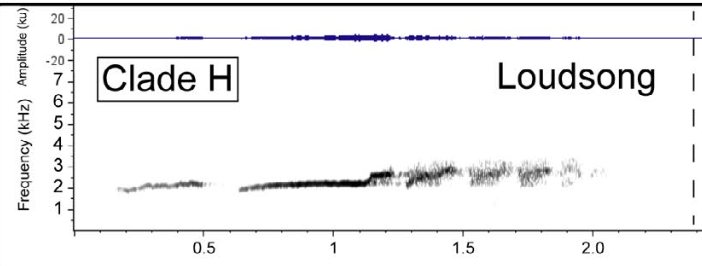
And
below is the loudsong of clade N (aequinoctialis group, but later
revealed to be the new species, cryptus):
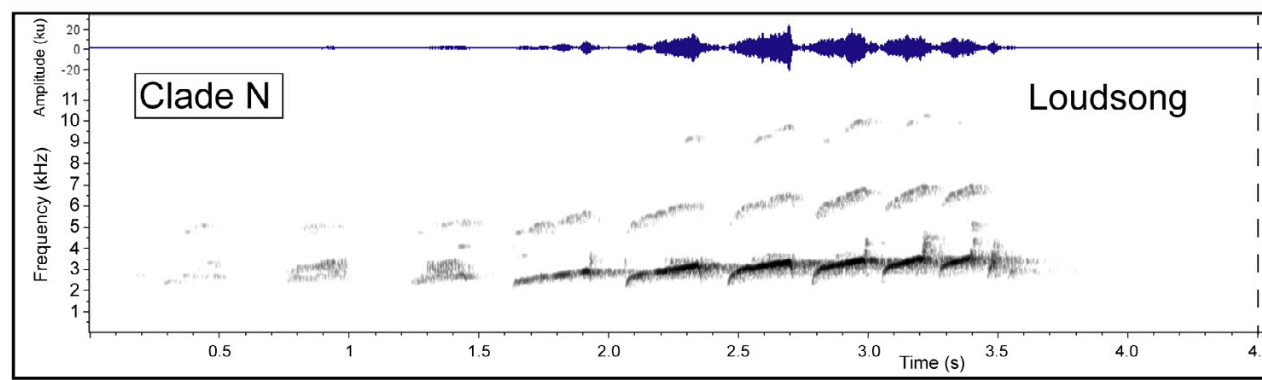
See
Simões et al. for detailed descriptions of the songs of all the clades – the
details are too lengthy to repeat here.
A PCA analysis of the measurements from the sonograms produced the
following result:
“Four
vocal measurements presented significant statistical differences across clades
and taxa (PF, PF1, PF2 and NN), but with no single group being unequivocally
diagnosed by any of the characters measured, given overlap in measurements
between two or more groups (Table S10). Nevertheless, a PCA analysis retaining
81% of the total variance in two first principal components (PC1: 53% and PC2:
28%), recovered along PC2 the two main vocal groups reported above, one
including clades D, H, I, J and K (hereafter called vocal group 1—VG1) and an-
other containing clades L, M and N, plus the samples attributed to R. o.
tamborensis and R. o. flavus (hereafter called vocal group 2—VG2;
Figure S4).”
Here
their Figure S4:
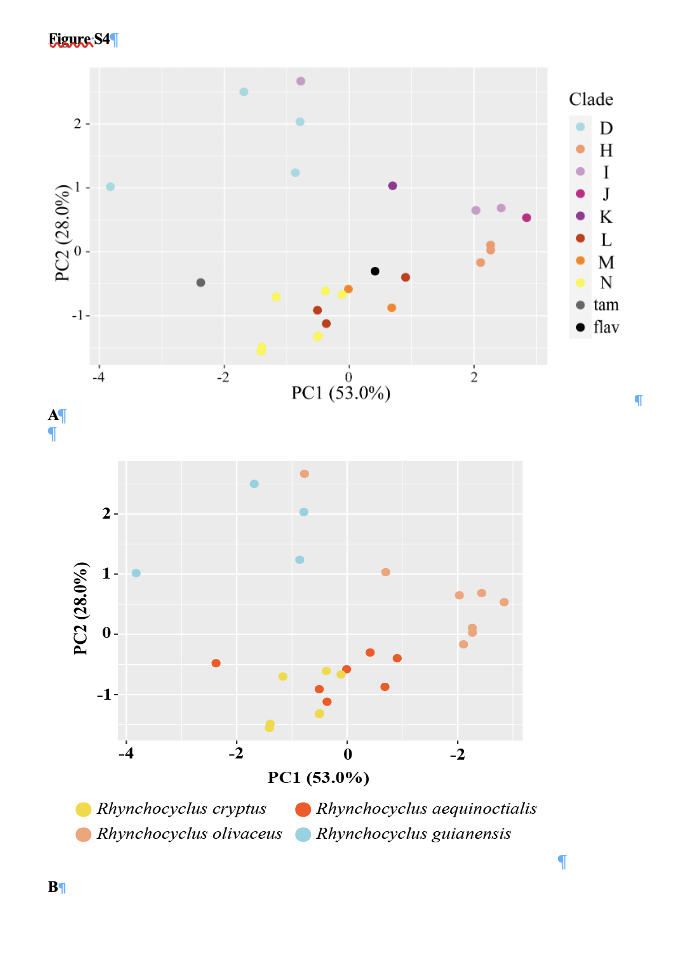
The
most unexpected finding in this project was that representatives of two of the
vocal groups are locally sympatric:
“Also,
our results demonstrated that clade N (belonging to VG2) overlaps broadly in
distribution with distantly related clades F and G (both belonging to VG1) in
different parts of south-western Amazonia (Figure 1), clearly indicating that
these lineages may have already acquired a significant level of reproductive
isolation consistent with their paraphyletic status, and current sympatry. This
sympatry in south-western Amazonia between different clades of R. o.
aequinoctialis is apparently mediated by occupancy of distinct habitat
types. Out of the 25 specimens sequenced attributed to R. o. aequinoctialis and
belonging to clades F, G and N, a clear pattern emerged: specimens from closely
related clades F + G (N = 9) were collected mostly in upland terra-firme
forest (N = 5), transitional forest (N = 2) and bamboo
patches (n = 2), whereas those in distantly related clade N (N =
16) were obtained from várzea seasonally flooded forest (N =
12), riparian forest (N = 2) and secondary forest (N = 1) (Table
S2). Our sampling revealed that sequenced specimens of distantly related clades
G and N are found within a few kilometres from each other in suitable habitats
in the Rio Branco capital area in the Brazilian state of Acre, with birds in
clade G associated with bamboo patches, and those in clade N with seasonally
flooded forests with varying levels of disturbance along the banks of the Rio
Acre (Figure 1; Table S2). We anticipate that a similar pattern of local
ecological replacement across upland and seasonally flooded habitats may also
occur between birds in clades F and N in the westernmost part of the Amazon
(Figure 1; Tables S2 and S3). These
genetically, vocally and ecologically divergent lineages of the Olivaceous
Flatbill lineages overlapping in south-western Amazonia are apparently
undistinguishable from a morphological perspective, when both plumage and
morphometric variation are considered (Table S8, Figures S2 and S3),
highlighting another remarkable case of cryptic diversification in the
Neotropics.”
”
Simões
et al. then interpreted all this in a taxonomic framework. First, they reasoned that the two major
lineages should be considered separate species, minimally, with the oldest name
for lineages D through K being olivaceus, and lineages L-N, aequinoctialis
(from Traylor “Peters” 1979: Rhynchocyclus olivaceus aequinoctialis
(Sclater) from Cyclorhynchus aequinoctialis Sclater, 1858, Proc. Zool.
Soc. London, 26, p. 70, type loc. Rio Napo, Ecuador). Then they proposed recognizing an additional
two species:
“However,
our data and analyses also clearly show that within these two major R.
olivaceus groups, there is still significant molecular and diagnostic vocal
variation allowing for the delimitation of additional species occupying
distinct biomes and river drainages across the Neotropics (Figures 1–3; Figure
S4; Tables S5 and S11). Namely, vocal and genetic patterns of variation allow
for the split of the major R. olivaceus and R. aequinoctialis groups
into at least two additional species level taxa each.”
Based
on that variation, they recommended the following treatment:
1. R. olivaceus
for clades H, I, J, K (Atlantic Forest region from Pernambuco to Rio; also
allopatrically in se. Amazonian Brazil E of the R. Tapajós. Includes sordidus.
2. R. guianensis
for clades D, E, F, G (Guianan Shield, N of the Amazon in Peru and Ecuador; S
of the Amazon from R. Jutai in Amazonas, Brazil west to Loreto, San Martín, and
Pasco, Peru; south of the Amazon from the R. Tapajós west to the R. Purús in
Pará, Amazonas, and Acre, Brazil. Note
that this includes several populations traditionally assigned to
aequinoctialis.
3. R.
aequinoctialis for clades L and M only (e. Panama and n. Colombia; nw.
Amazonia in ne. Ecuador; presumably also in e. Colombia. Includes bardus and, provisionally,
the subspecies mirus, tamborensis, flavus, and jelambianus.
4. R. cryptus
sp. nov. for clade N
The
latter is formally described as a new species in the paper, which see for all
the details, including registry in ZooBank and deposition of the type specimen,
from Acre, at Museu Goeldi. The new
species is a varzea specialist that like some other varzea species occurs also
in human-degraded habitat. It is
widespread in the Inambari area of endemism (south of Amazon from e. Peru east
to the Madeira in Brazil, and south to northern Bolivia); where sympatric with guianensis,
occurs in varzea forest whereas guianensis is in adjacent terra firme
forest.
I
attempted to map these four distributions using the localities mapped for each
clade in the map above on the map below.
Apologies in advance to Simões et al. for any mistakes – corrections
welcomed. Note that the situation in e.
Colombia, the R. Negro region of Brazil, and Amazonian Venezuela is unclear to
me.
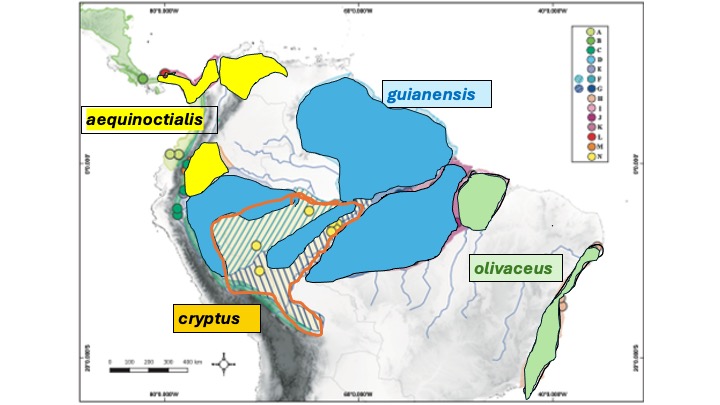
Discussion
I
spent more time on this proposal than any in 25 years of writing proposals for
SACC. This is a complex situation with
surprising and perhaps anomalous results.
Simões et al. did a very good job of putting this all together,
including helpful Supplemental Information, and a huge amount of work went into
this project.
That
there are more than one species hidden in a widespread Neotropical tyrannid is
of course no surprise. Nonetheless, I
have some concerns. First, the
biogeographic pattern is unique, as far as I can tell, especially for the four
disjunct populations grouped under aequinoctialis. Some of that may be due to incomplete sampling. Second, the taxa are essentially
undiagnosable in terms of plumage and morphology. Third, although the vocalizations look to be
diagnostic, the samples of loudsongs are small, and the PCA analysis seems to
show that they overlap or nearly so.
Fourth, given that the species tree typology conflicts with the Harvey
et al. topology that has much better genetic sampling, how then are we supposed
to trust the finding that R. rufipectus is embedded to the olivaceus
clade? If the latter were correct, then
of course division of traditional olivaceus into at least two species
would be required.
For
voting purposes let’s break this down as follows, with YES/NO votes on the
following
A. Treat R.
aequinoctialis as circumscribed by Simões et al. a separate species. Even if the position of R. rufipectus
is questionable, a case can be made for the split based on voice.
B. Treat R. guianensis as a separate
species.
C. Treat R. cryptus
as a separate species. This would be
required by the sympatry of cryptus and guianensis in sw.
Amazonia, but whether cryptus should be treated as a separate species
from R. aequinoctialis needs to be considered.
I
do not have any firm recommendations. It
seems to me that Simões et al. have discovered a fascinating system, and I look
forward to comments by others.
Literature
Cited:
BOESMAN, P. 2016p . Notes on the vocalizations of
Olivaceous Flatbill (Rhynchocyclus olivaceus). HBW Alive Ornithological
Note 120. In: Handbook of the Birds of the World Alive. Lynx Edicions,
Barcelona. https://doi.org/10.2173/bow-on.100120
SIMÕES,
C. C., P. V. CERQUEIRA, P. PELOSO, AND A. ALEIXO. 2022.
Integrative taxonomy of flatbill flycatchers (Tyrannidae) reveals a new
species from the Amazonian lowlands. Zoologica Scripta 51:
41-57.
Note
on English names:
BOW uses Western Olivaceus Flatbill for aequinoctialis and Eastern
Olivaceous Flatbill for olivaceus.
Simões et al. retained parental Olivaceus Flatbill for olivaceus,
and used Guianan Flatbill for guianensis, Equinoctial Flatbill for aequinoctialis,
and Cryptic Flatbill for cryptus. We will have to do a separate SACC proposal on
English names depending on which taxonomy, if any, we adopt.
Van Remsen, May 2025
Voting Chart: https://www.museum.lsu.edu/~Remsen/SACCPropChart1044+.htm
Comments from Areta: “This was my AviList/WGAC vote:
‘These papers with a mixture
of scientific names and letters for clades are so exasperating that I want to
commit arson.
‘I don´t think that the 2-species arrangement is the
best possible one, but I also see that there is room for nuanced taxonomic
variants here.
‘The bioacustic analyses
are far from convincing. However, in examining the spectrograms visually, I
must say that clades DEFG and HIJK seem to differ markedly in vocalizations
(the former having a descending series of overslurred
notes, the latter a series of upswept notes). This suggests that R. guianensis may also be split from R. olivaceus. Their PCA provides some support for this, although
not overwhelming. Note also that the Amazon and Tapajos rivers separate the guianensis from the olivaceus groups. The deep split between clades G (of guianensis) and adjacent K (from olivaceus) contrasts markedly with the shallow
differentiation between the widely allopatric populations of the Atlantic
forest (clade H) and the south-east Amazonian populations (IJK).
‘On the other hand, the species-level status of cryptus (clade N) seems more debatable given the vocal
similarities to the relatively deeply split from aequinoctialis (clades LM) and is to me a borderline case that
needs more refinement, but which may be correct.
‘I vote for the following: A) Split R. aequinoctialis (with cryptus as a subspecies, unless someone finds a compelling
reason to also split cryptus), and B) Split R. guianensis.
‘Check the bioacoustic PCA and genetic distances
between clades in the following suppl mat:
zsc12519-sup-0004-figs4.docx
zsc12519-sup-0006-supinfo.pdf
"In terms of SACC voting this would
be:
A)
YES, recognize R. aequinoctialis (clades
LMN), including cryptus as a
subspecies
B)
YES, recognize R. guianensis (clades
DEFG; descending series of overslurred
notes)
C)
NO, include cryptus (clade N) as a
subspecies of aequinoctialis (but if
someone provides good arguments to recognize it, I am willing to reconsider)
D)
This would mean that I also support a newly circumscribed R. olivaceus (clades HIJK; series of upswept
notes)”
Comments
from Stiles:
“YES to all splits, but I agree with Nacho that on present evidence, cryptus
may best be considered a subspecies of aequatorialis.”
Comments
from Robbins:
“After spending nearly two hours over a two day period reviewing data
associated with the Simões et al. (2022) publication,
I fully understand why Van stated that he spent more time on this proposal than
any other (!) AND why he did not make a recommendation. I also share Nacho’s frustration with aligning
and digesting data sets based on a letter code for each clade. Moreover, if you also do a deep dive into the
vocalizations, i.e., going to Xeno-canto and Macaulay Library, you almost
certainly will reiterate Van’s remarks.
“It
is very impressive the amount of work that Simoes et al. put into elucidating
this very cryptic complex that is not dissimilar to Tolmomyias. Major
kudos to them for drastically increasing our understanding of this group.
Clearly, there is more than one species in the currently recognized olivaceus
complex; however, as with any study that involves such a difficult group there
are a few issues that still need to be addressed. Given that plumage morphology
does not offer insight to species limits within this complex, my comments are
focused on genetics and vocalizations.
“1. Genetics: As
Van pointed out, it is of concern that the results of the Harvey et al. (2020)
contradict that of Simões et al. with the
relationship between pacificus and brevirostris. But because the
Harvey et al. study only included one sample of olivaceus (nominate from
southeastern Brazil) nothing more can be concluded comparing the two data sets.
Despite the relatively large uncorrected distances in the mitochondrial genes
among taxa, a whole genomics perspective is needed including sequencing the
holotype of aequatorialis (see below).
“2. Vocalizations.
Naturally, since the publication of Simões et al.
(2022) there are more vocalizations available online. Without going into too
much detail, please note that in Figure 4 the example of clade N, i.e., what
they are calling the new species, cryptus, is ML 75277 (also see a
complete list of vocal samples in their Supplementary material). That recording
and others allocated to clade N are striking similar to a more recent recording
(https://ebird.org/checklist/S197298853) from Reserva Munay Suyu, at the base of the
Andes in Prov. Napo, Ecuador, i.e., that locality appears to be in terra
firme.
“So,
this raises a couple of questions. If the Napo bird isn’t an example of cryptus
then the loudsong of aequatorialis is quite similar to cryptus.
If the Napo recording is of cryptus and given the holotype of aequatorialis
(sensu stricto) is from the Upper Río Napo, Prov. Napo, Ecuador, and Simões et al. apparently didn’t sample the holotype (see
Supplemental material), then the question needs to be asked, could the new taxon actually be aequatorialis? That would result
in the taxon flavus (it has priority) to be applied to cis- and
trans-Andean populations that Simões et al. consider
subspecies under aequatorialis. Regardless, the holotype of aequatorialis
needs to be sequenced to clarify what name should be applied to what group.
“The
above along with the truly unusual biogeographic pattern of proposed species
limits by Simões et al. makes me question just how
many species are involved. Again, I have no doubt that more than one species is
involved, but because of what I have highlighted above, at this point, I can
only support part B, elevating guianensis (there is no issue with that
name being applied to at least birds north of the Amazon) to species level
because of the unique loudsong. Finally, if aequatorialis and cryptus
are considered the same species, I would also vote for recognition of a third
species while waiting for information that clarifies what I have outlined
above.”
Comments
from Bonaccorso:
“NO to all. Definitively a fascinating
system, but very complex. I am not sure we have the necessary data to make a
decision now.”
Comments
from Zimmer:
“I have no doubt that multiple species are currently nested within this
complex, given vocal differences between populations that I have noted during
the course of my own fieldwork, and fortified by the recent published genetic
data. Frustratingly, however, a review
of archived recordings only partially supports the taxonomic treatment
recommended by Simões et al. (2022). I would agree that Atlantic Forest
populations (nominate olivaceus) and allopatric sordidus (from SE
Amazonian Brazil, east of the R. Tapajós) should be treated as a single
species, distinct from all other taxa in the complex. I also agree that the
remaining taxa can be sorted, on a coarse-grained scale, into one of two main
vocal groups based upon major differences in loudsongs (descending series
versus ascending series of notes, albeit with a lot of variation in pace,
frequency, change of pace, etc.), as per Boesman (2016). After that, however, the treatment
recommended by Simões et al. (2022) looks muddled to
me. Here are the next two recommended
parts of their taxonomic framework, copied from the Proposal:
“2. R. guianensis for clades D, E, F, G
(Guianan Shield, N of the Amazon in Peru and Ecuador; S of the Amazon from R. Jutai in Amazonas, Brazil west to Loreto, San Martín, and
Pasco, Peru; south of the Amazon from the R. Tapajós west to the R. Purús in
Pará, Amazonas, and Acre, Brazil. Note that this includes several populations
traditionally assigned to aequinoctialis.
“3. R. aequinoctialis for clades L and M
only (e. Panama and n. Colombia; nw. Amazonia in ne. Ecuador; presumably also
in e. Colombia. Includes bardus and, provisionally, the subspecies mirus, tamborensis,
flavus, and jelambianus.
“The
Guianan Shield populations (guianensis) definitely belong in Vocal
Group 1 (the “descending series”), as evidenced by the sonogram of Clade D
presented in the Proposal. I reviewed
audio recordings of songs of guianensis from the North Bank of the
Amazon, and east of the R Negro (Manaus region in Amazonas, Brazil; Bolívar,
Venezuela and French Guiana), and all of them fell into Vocal Group 1.
Recordings from south of the Amazon, and from the Madeira-Tapajós interfluve (e.g.
Ji-Paraná, Rondônia; Itaituba, Borba) were also
assignable to Vocal Group 1. However,
none of the recordings from south of the Solimões and west of the R Madeira
that I reviewed, were of the descending series Vocal Group 1 type. Rather, they were all some variation on the
“ascending series of notes” or Vocal Group 2 type. This applied to multiple recordings from
Acre, Brazil; Bolivia; Madre de Dios, Peru; and, to the north, from various
locales in Ecuador and western Colombia, as well as a bunch of recordings from
Panama. As far as I can tell,
populations from all of these locales west of the Madeira, belong to the Vocal
Group 2, and, at least on the basis of loudsongs, should be considered as part
of the aequinoctialis-group. So,
although the two major vocal groups, as defined, appear to be on the mark, some
populations seem to be allocated to the wrong vocal group in the taxonomic
framework recommended by Simões et al. (2022). I could not locate enough archived recordings
of songs from N Venezuela, E Colombia and NW Amazonian Brazil (north of the
Solimões & west of the R Negro) to assess where those populations would map
out vocally, so that’s one big question mark in my mind. Another involves variation within each of the
two primary Vocal Groups, how significant that variation is, and how it
corresponds to named taxa. For instance,
it’s hard for me to assess the significance of loudsong differences between sp.
novum (cryptus) and the rest of Vocal Group 2, without a robust
analysis. There’s also the question of
differences in vocalizations other than loudsongs. Nominate olivaceus from the Atlantic
Forest seems to have 2 commonly given song types, one of which is a descending
series of notes, which, although different, is at least in the same ballpark as
the songs of guianensis, whereas the other song type is much shorter,
differently patterned, and qualitatively different in tone. Ssp. bardus,
from Panama, although recognizably of the same Vocal Group 2 as aequinoctialis,
differs in the frequency with which it sings loudsongs. In my experience, these Panamanian birds only
rarely give the loudsong, but call (a short, buzzy “zzheeyp”)
frequently. So, lots of questions still, although I agree with many of the
broader conclusions of Simões et al. (2022). For SACC voting purposes, this would be my
vote:
A)
YES,
recognize R. aequinoctialis as a separate, polytypic species (to include
bardus, as well as cryptus among other
taxa that fall into Vocal Group 2, with the ascending series of notes).
B)
YES,
recognize R. guianensis as a separate species, corresponding with Vocal
Group 1 (descending series of notes).
C)
YES,
tentatively, to treating R. cryptus as a distinct species from aequinoctialis,
based mainly upon parapatric distribution as delimited by habitat type,
genetics, and some vocal distinctions (the extent and significance of which,
have not been demonstrated).
D)
This
isn’t listed in the voting framework, but Nacho included it, and I think it
should be stated, that YES, I support a newly circumscribed R. olivaceus
(that includes sordidus as a subspecies), the members of which share a 3rd
song type (short series of upswept notes with a flutier, more musical, and less
harsh or frequency-modulated quality to the notes).
Comments
from Lane:
“Wow, this is a case I have been avoiding because of its complexity! But an
interesting development in the past week has piqued my interest and helped me
understand a little better what may be going on.
“I
guided a tour in the Andean foothills of the Alto Mayo (dept San Martín), Peru
and recorded a mystery sound (ML640978343, ML640979054) that I assumed was an
antbird song. I played it to Bret Whitney last week, and he identified it as Rhynchocyclus
olivaceus, which floored me, as I have only heard the accelerating rising
note song in Peru (which was why I was so confused by the Simões
et al. results!). Bret then shared with me a recording he had made in the past
month on the Cordillera Escalera (the eastern boundary of the Alto Mayo
valley), a site where I too have encountered the bird, and in relistening to my
recording from there (ML622396166), I was interested to
hear that I had heard the descending song type, although unable to document it
in the recording! Further, one of the specimens used by Simões
et al. that placed Clade E in Peru was an LSU specimen collected in the
Escalera by Tristan Davis! So, it seems to me, this voice type is present in
Peru in the foothills, but these are part of the Guianan Clade D and not sister
to the adjacent lowland birds with the other song type (Clade N). So, two song
types are in Peru, but I do not see evidence of them being sympatric (yet).
Quite possibly they co-occur across Amazonia, perhaps separating in terra firme
vs varzea habitats where they overlap, a pattern that is emerging among some
other species complexes, and the fact that most Peru-centric researchers are
familiar with Clade N voice (see: https://media.ebird.org/catalog?birdOnly=true&taxonCode=olifla2&mediaType=audio&view=grid®ionCode=PE&sort=obs_date_asc), and have written
Clade E off as something else (as I have!), could mean the latter is slipping
under the radar in Peru. Mark makes a good point about that the holotype of aequinoctialis
should be sampled to confirm that it is indeed the same beast as Clade M, but
the voice of M and N seem distinctive enough to me that I can agree that they
may be best considered two species, and I don’t think the name aequinoctialis
can be placed on Clade N. There is one recording from Brazil on the right back
of the Rio Jurua (XC90673) that documents the
Clade E song type south of the Solimões and west of the Madeira.
“So,
that is a long-winded way of saying I think I have picked my way through this
complex issue and will vote the following: 1) YES. 2) NO. I believe it may be
better to consider guianensis as part of olivaceus given the
vocal similarity and the phylogenetic placement. 3) YES. 4) YES. This results
in a 3-way split: a northwestern R. aequinoctialis, a western
Amazonian R. cryptus, and a more widespread R. olivaceus
(from Andean foothills to the Guianan shield and SE Amazonia and a disjunct
population in the Atlantic forest).”
Comments
from Claramunt:
“Difficult case. The molecular data are consistent with the four-way split
proposed, but it is likely mostly a mitochondrial signal. I would like to see
what the nuclear genes show. The vocal variation is rich and alleged to be
geographically structured and totally congruent with the four main
mitochondrial clades, but I don’t see in the paper a figure showing the
distribution of songs or an evaluation of whether song variation is discrete or
continuous. The lack of significant plumage variation makes things even more
difficult. Therefore, I vote conservative. I think that splitting the complex
into two species is the best option at this point: olivaceous (including
guianensis) and aequinoctialis (including cryptus). This
taxonomy would separate two strongly supported clades with contrasting songs
patterns (ascending versus descending), that are sympatric in W Amazonia.
Further splits of the complex are just not completely clear, in my opinion.
Specifically:
“A.
YES to treat R. aequinoctialis (and related subspecies) as a separate
species.
“B.
NO to treat R. guianensis as a separate species. I am not convinced that
song variation is discrete. Recordings on either side of the Tapajos are very
similar, the main difference being only a faster pace in the W (compare https://xeno-canto.org/66668 and https://xeno-canto.org/147429)
“C.
NO. Songs are fairly like those of aequinoctialis and an evaluation of
whether variation is discrete or continuous would be required to make
inferences from the data. In the meantime, the new taxon can be treated as a
subspecies of aequinoctialis, separated from the sympatric olivaceous
guianensis.
“Hard
to say for sure because the supplemental figures are barely labeled, let alone
having a caption, but this seems to be the map with the proposed species limits:”
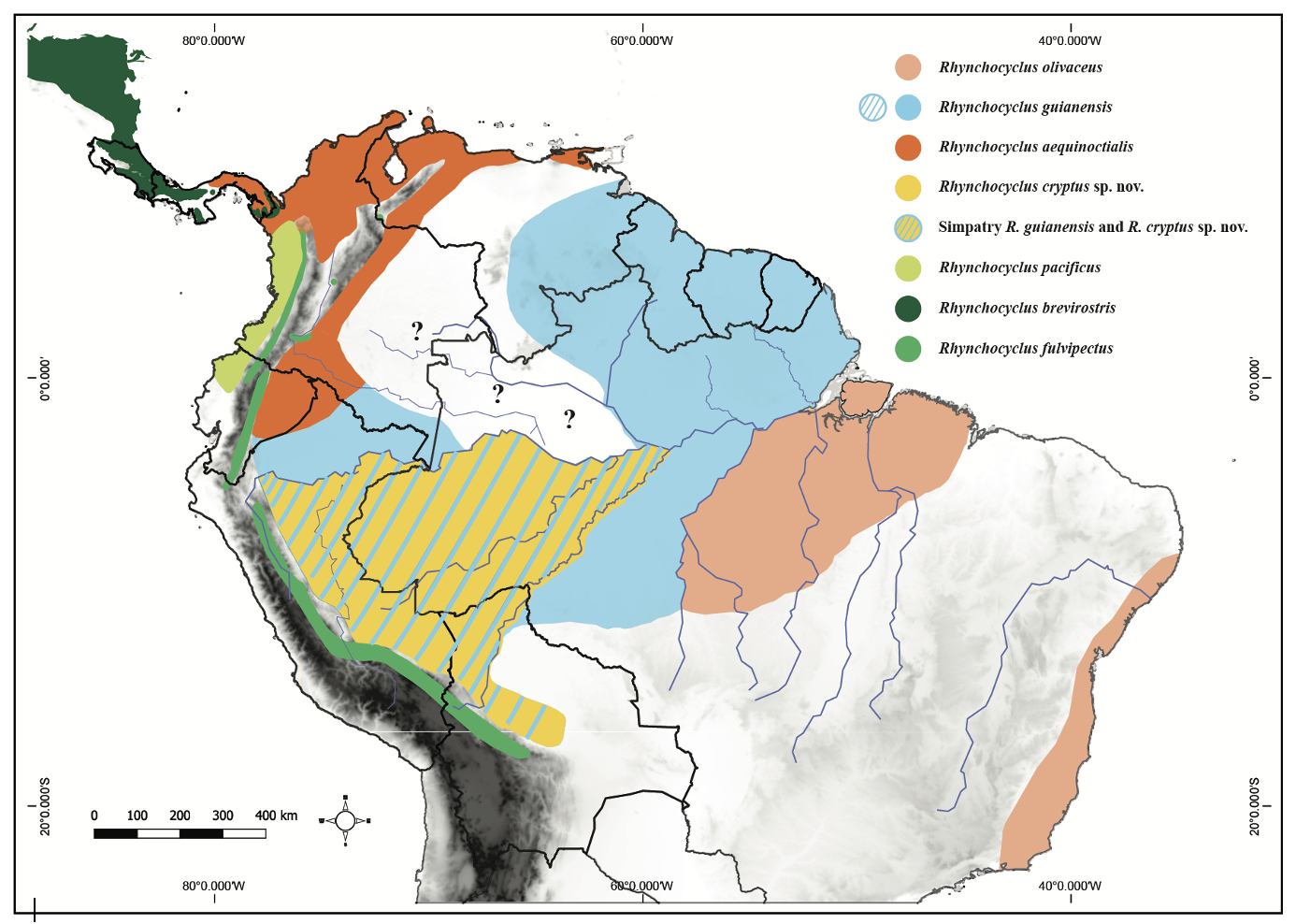
Comments
from Remsen:
“I am strongly persuaded by Santiago’s reasoning above and cannot add anything
to it, so A. YES. B. NO. C. NO.”