Proposal (1060) to South
American Classification Committee
Recognize Aphrastura subantarctica
as a separate species from A.
spinicauda
Analysis
Aphrastura spinicauda is currently considered polytypic, with a widespread nominotypic subspecies spinicauda, and two island taxa
restricted to Chile: bullocki from
Mocha Island and fulva from Chiloé.
Rozzi et al. (2022a,b) described the Diego Ramirez population of Aphrastura spinicauda as a new taxon at
the species level under the name of Subantarctic Rayadito (Aphrastura subantarctica). Their conclusions are based on
morphological data of 117 individuals (three populations) and genetic data of
the mitochondrial cytochrome b gene (120 samples from 20 localities) and 12
nuclear microsatellite markers from 153 individuals of five Aphrastura populations (Figure 1; Rozzi
et al. 2022a).
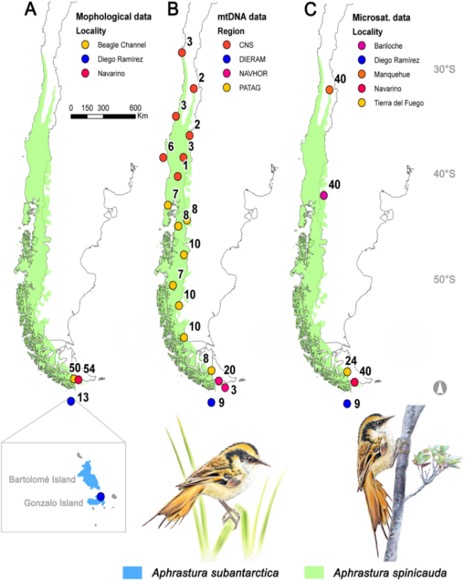
Figure 1. Study areas for the
morphological and genetic characterization of Aphrastura spinicauda and A.
subantarctica. The distribution range of the nominal species is shown in
light green, and the new taxonomic group from the Diego Ramírez Archipelago in
light blue. (A) Sampling sites for
morphology. (B) Sampling sites for
mtDNA. (C) Sampling sites for
microsatellite markers. The numbers correspond to the sample size. From Rozzi
et al. (2022a).
The taxon
was described in two articles (Rozzi et al. 2022a,b), because the first one
(Rozzi et al. 2022a) did not comply with the standards of The International
Code of Zoological Nomenclature (ICZN):
“Diagnosis: Morphology.— Typical Aphrastura structure
with rounded wings, and an idiosyncratic tail morphology. Aphrastura’s
distal third of the inner web of the rectrices is abruptly and deeply excised,
giving the tips of the feathers a thornlike appearance. No other genus in the
family has a similar tail structure. Aphrastura
differs in these morphological characters from the phylogenetically closest
related genera in the subfamily Synallaxinae present in southwestern South
America: Leptasthenura and Sylviorthorhynchus. In contrast to Aphrastura, Leptasthenura’s tail is not abruptly and deeply excised at the
distal portion of the inner web of the rectrices; in Sylviorthorhynchus, the rectrices are denuded of barbs. A. subantarctica differs from A. spinicauda, in having on average a
larger and heavier body (~25%), a larger beak (~15%), a larger tarsus (~5%),
and a shorter tail (~16%) (Fig. 2 in this proposal). The primaries and
secondaries are greyish on the ventral side with whitish edges; the central rectrices
are dark grey on the ventral side, but do not differ between the two species.”
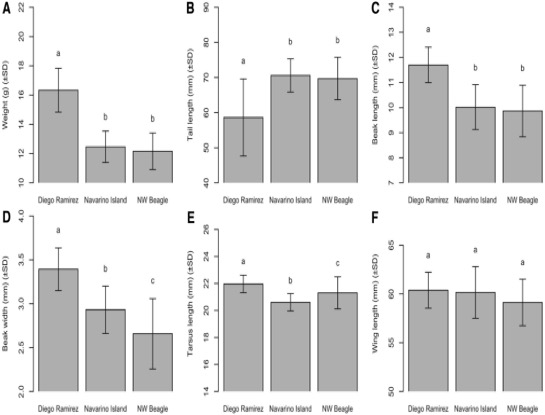
Figure 2. Comparison of body weight
(A), tail length (B), beak length (C), beak width (D),
tarsus length (E), and wing length (F) among Aphrastura populations. Metrics (means ± SD) of birds from the
northwest arm of the Beagle Channel (NW Beagle, n = 50), Navarino Island
(n = 54), and the population of the proposed new species A. subantarctica on Gonzalo Island, Diego Ramírez (n = 13).
Lowercase letters indicate statistically significant differences at an alpha of
0.05. From Rozzi et al. (2022a).
Morphology
Rozzi et al.
(2022a) measured a total of 117 adult individuals of Aphrastura in the NW Beagle Channel (n = 50), Navarino Island (n =
54), and Diego Ramírez (n = 13). Morphological differences among populations
were statistically supported for weight, tail length, tarsus length, beak
length, and beak width (all p < 0.05), but not for wing length (p >
0.05). Birds from the Diego Ramírez population were significantly heavier and
larger (with a longer and wider bill and longer tarsi), but they had a
significantly shorter tail than birds from the other two populations. The PCA
analysis shows that the Aphrastura populations
of the Beagle Channel and Navarino Island overlap in body dimensions, whereas
the individuals of the Diego Ramírez population form a clearly separate cluster
(Rozzi et al. 2022a).
Phylogeographic patterns:
Presented in
Rozzi et al. (2022a): Overall mitochondrial genetic diversity was low,
revealing a short genealogy of the cytb gene. Genetic diversity was larger in
populations from southern Chile between 42 and 53° S (from Chiloé to Punta
Arenas), compared to that of populations in the center and the north of the
distribution, and also of the populations in the extreme southern part of the
distribution (Navarino and Horn islands). Noticeably, based on pairwise FST and
Ф values, the Diego Ramírez population is strongly and significantly separated
from all other populations. Individuals sampled from Diego Ramírez shared the
exact same haplotype, which differed by one mutation from the most dominant
haplotype found in A. spinicauda. The
Diego Ramírez haplotype is also present on Horn Island (1 out of 3 individuals)
and at low frequency in Navarino Island (1 out of 20 individuals) (Fig. 3 from
Rozzi et al. 2022a).
The two
articles describing the new taxon do not present a phylogenetic analysis or any
explicit species delimitation analysis.
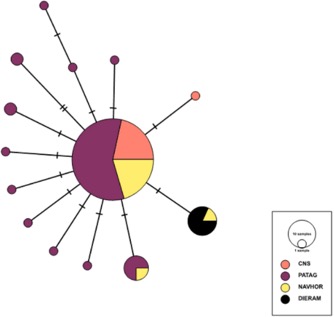
Figure 3. Mitochondrial haplotype
network of Aphrastura geographic
groups. Each circle in the network corresponds to a different haplotype, the
size of the circles corresponds to haplotype frequencies, and the colors correspond
to the different geographical groups. CNS
Central, north and south, PATAG Patagonia
(Chiloé to Tierra del Fuego), NAVHOR Navarino
and Horn islands, DIERAM Diego
Ramírez (representing the proposed new species A. subantarctica). From Rozzi et al. (2022a).
Population genetic structure:
Presented in Rozzi et al. (2022a):
For the PCA analysis of the five sampled populations, retained the first 43
dimensions, which explained 85% of the total genetic variation. A plot with the
first three components (~ 26% of variation explained) showed that Navarino
Island and the southern continental populations (Bariloche and Tierra del
Fuego) formed a homogeneous group (Fig. 4). The northernmost continental
population (Manquehue, central Chile) was slightly separated, but relatively
well mixed with the southern continental group (Fig. 4). In contrast, the
Diego Ramírez population appeared well isolated from all other sampled
localities, regardless of the combination of principal components being
examined (Fig. 4). According to the AIC values obtained from the
‘snapclust’ method, the optimal number of genetic clusters within the group of
sampled individuals was three. This estimate is congruent with the number of
clusters emerging from the PCA analysis: (1) a central cluster, comprising the
Manquehue population in the center of the species’ distributional range; (2) a
southern cluster, composed of Bariloche, Tierra del Fuego Island, and Navarino
Island populations; and (3) Diego Ramírez (Rozzi et al. 2022a).
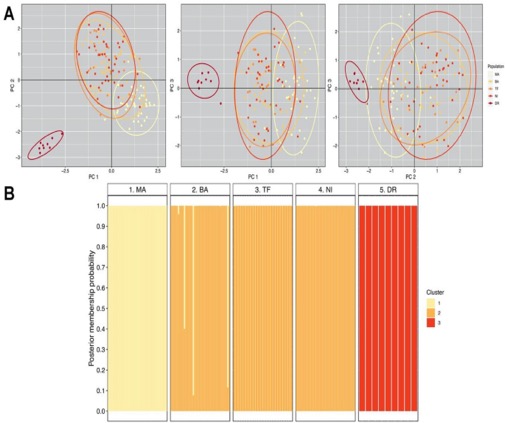
Figure 4. Genetic variation among
five populations of Aphrastura, based
on 153 individuals genotyped at 12 polymorphic microsatellite loci. (A) Scatterplot from the first three
principal components that explained 25.5% of the genetic variance. 95% CI ellipses
are shown. (B) Individual assignment
to genetic clusters for the five sampled populations. Bars represent individual
posterior membership probabilities to each of the three genetic clusters found
using the ‘snapclust’ method. MA Manquehue,
BA Bariloche, TF Tierra del Fuego, NI Navarino
Island, DR Diego Ramírez
(representing the proposed new species A.
subantarctica). From Rozzi et al. (2022a).
Vocalizations
There is no detailed comparison of
the vocalizations of Aphrastura subantarctica
concerning the rest of the populations/subspecies, only a general mention
that indicates (see Rozzi et al. 2022a,b):
"Across the entire range of Aphrastura
spinicauda, individuals respond to intruders near their nest sites with alarm
calls. In the continental populations, mobbing calls have minimum and maximum
frequencies of 2.82 and 13.01 kHz, respectively, with at least six notes per
second in central and southern Chile (Ippi et al. 2011). However, based on a
previous record in 2001, it seems that minimum and maximum frequencies of
mobbing calls of Aphrastura subantarctica are lower, with
the same number of notes (see Imberti 2011). While these preliminary
observations have to be confirmed by future studies, the low call frequencies
of Aphrastura on Diego Ramírez could be related to the high ambient
noise, as well as their larger body size (Mikula et al. 2021)."
A work by Ippi et al. (2011)
reported that vocalizations among the five populations showed some variation in
the repetitive trill. In contrast, no differences were found between alarm
calls and loud trills. Variation in repetitive trills among populations and
forest types suggests that sound transmission may explain vocal differences in
suboscines. Acoustic differences help distinguishing subspecies bullocki from spinicauda and fulva, but
not the latter two subspecies from each other. Ippi et al. (2011) suggests that
the geographical differentiation in vocalizations observed among Thorn-tailed
Rayadito populations could result from different ecological pressures.
Vocalization examples and comparison
across the range of Aphrastura spinicauda:
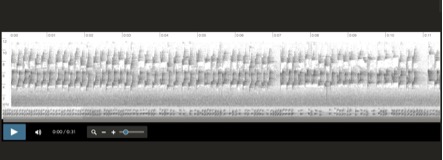
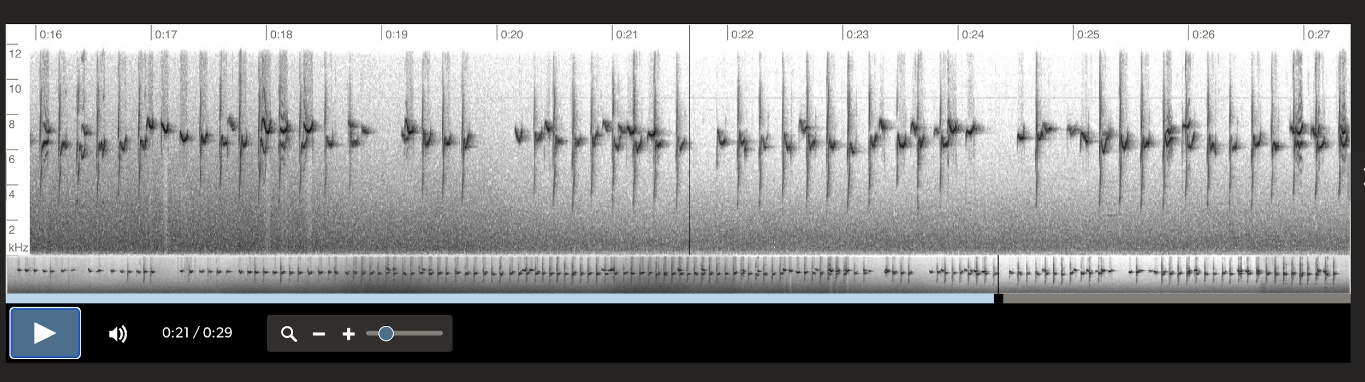
Aphrastura spinicauda, MN Cerro Ñielol,
Temuco, Chile, 11-8-2012 Heraldo Norambuena Ramirez
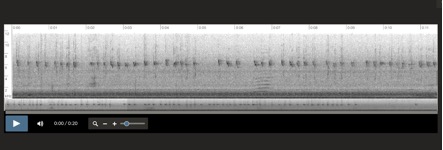
Aphrastura spinicauda, PN Los Glaciares,
Argentina, 6-1-2020 Gabriel Leite
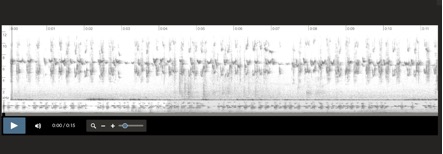
Aphrastura spinicauda, PN Radal
Siete Tazas, Maule, Chile 2-11-2024 Vicente Pantoja Maggi
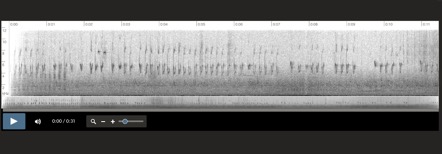
Aphrastura spinicauda, Rio Negro, Chaitén,
Chile 20-9-2021 Freddy Sepulveda
Aphrastura spinicauda, Islas Diego Ramirez, Chile
6-1-2001 Santiago Imberti
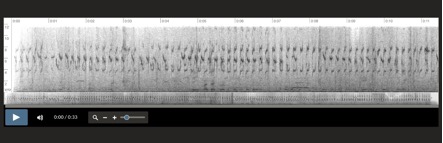
Aphrastura spinicauda, Islas Diego Ramirez, Chile 6-1-2001 Santiago Imberti
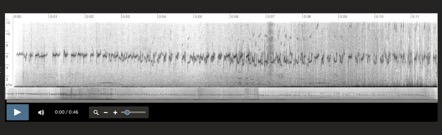
Aphrastura spinicauda, Islas Diego Ramirez, Chile 6-1-2001 Santiago Imberti
Conclusion
Rozzi et
al.'s (2022a,b) approach to describing a new taxon was based on population
genetics. Differences in the cytb marker only show a single mutational step
between widely separated geographic areas. As expected with macrosatellite
markers, divergence is more substantial using this source of information.
However, the cluster analysis not only shows a cluster for Diego Ramírez, but
also a cluster for the northernmost part of Maquehue. This result could suggest
an identification of genetic structuring rather than a speciation event. Due to
the winds found on subantarctic islands, short tails are apparently favored
(there are no trees to climb and the tail acts as a sail in the wind) and short
wings in relation to mass/size (which is why birds on these islands tend to
become flightless).
The habitat used by Aphrastura in Diego Ramirez does not differ
from that found in Cape Horn and the Mitre Peninsula, Tierra del Fuego
(Argentina) where rayaditos are locally common at great distances from forest.
Here, rayaditos are locally abundant in areas with tussock grass and low scrub,
where they rarely fly and move close to the ground in dense vegetation avoiding
the omnipresent windy conditions. Rozzi et al. make no attempt to compare any
aspect (morphological, behavioural, plumage or vocal) with these closest
populations of Aphrastura that occur in the same habitat which we
believe should have been a fundamental starting point of their study.
Furthermore, a comparison of the closest forest populations and those occurring
in stunted Nothofagus antarctica would be desirable.
Although the
frequency in vocalizations (compared to a single set of recordings) seems to be
slightly lower, this negligible difference can be attributed to other factors
rather than be considered a factor to separate this population into a different
species.
The
structure recovered by the analysis of microsatellites (which portray very
recent phylogenetic information and are thus of limited utility when assessing
species limits) might be explained by and endogamic population in Diego
Ramírez, perhaps in part due to the difficulty of crossing from the continent
to the island.
The
morphological differences might be adaptive, as the cold and wind-barren Diego
Ramírez islands would exert pressure favoring a larger size and a reduced wing
length in relation to weight/size, while the shorter tail could be related to
the lack of trees and the advantage of not acting as a "ship sail".
We note that the diagnosis does not include any plumage or vocal feature, but
rests exclusively in morphometric differences. Although we do not dispute that
these differences exist, a broader comparison to understand how the geographic
variation is structured in Aphrastura
spinicauda would have provided a much better yardstick than limiting the
comparison to the southernmost populations. The lack of trees in Diego Ramírez
provides a drastic ecological contrast to the forest places in which A. spinicauda lives in most of its
distribution, however, populations in the Hornean Islands and the Mitre
Peninsula use tussock (Poa flabellata
grasslands) and shrub lands, showing that Aphrastura
is quite plastic. The subspecies fulva
from Chiloé, with its striking plumage differences from the continental forms,
indicates that Aphrastura populations
can exhibit marked phenotypic differences without a need for stringent
geographical barriers. Finally, vocalizations should be properly studied, even
if alarm calls are shown to be lower pitched in subantarctica than in the other taxa, this could just be an
incidental byproduct of size, and not in itself evidence for species status of
the Diego Ramírez population. In sum, we think that the data provided by Rozzi
et al. (2022a, b) is more consistent with subantarctica
being a subspecies of spinicauda.
Recommendation
We recommend
a NO vote. The reduced to null levels of genetic differentiation in a single
mtDNA marker (cyt b) coupled to a lack of vocal differences in calls (and
unknown, but apparently trivial levels of vocal differentiation in song; pers.
obs.) indicate to us that subantarctica
can be a good, mildly differentiated subspecies of A. spinicauda but not a separate species from it.
References
Imberti, S. (2001). Internet Bird
Collection: horn-tailed Rayadito (Aphrastura spinicauda).
https://macaulaylibrary.org/asset/204019791
Ippi, S., Vasquez, R. A., van
Dongen, W. F. & Lazzoni, I. (2011). Geographical
variation in the vocalizations of the suboscine Thorn-tailed Rayadito Aphrastura spinicauda. Ibis 153, 789–805.
Mikula, P. et al. (2021). A
global analysis of song frequency in passerines provides no support for the
acoustic adaptation hypothesis but suggests a role for sexual selection. Ecol.
Lett. 24, 477–486.
Rozzi, R.; Quilodrán,
C.S., Botero-Delgadillo, E., Napolitano, C., Torres-Mura, J.C., Barroso, O., Crego,
R.D., Bravo, C., Ippi, S., Quirici, V., Mackenzie, R.,
Suazo, C.G., Rivero-de-Aguilar, J., Goffinet, B., Kempenaers,
B., Poulin, E., Vásquez, R.A. (2022a). «The Subantarctic Rayadito (Aphrastura
subantarctica), a new bird species on the
southernmost islands of the Americas». Scientific
Reports. 12(1): 13957. ISSN 2045-2322. doi:10.1038/s41598-022-17985-4.
Rozzi, R., Quilodrán, C.S., Botero-Delgadillo,
E., Crego, R.D., Napolitano, C., Barroso, O., Torres-Mura,
J.C., Vásquez, R.A. (2022b). «El Rayadito subantártico: disponibilidad del binomio Aphrastura subantarctica (Passeriformes, Furnariidae)». Boletín
Museo Nacional De Historia Natural 71(2):
9-15. ISSN 2045-2322. doi:10.54830/bmnhn.v71.n2.2022.222
Heraldo V. Norambuena, Juan I. Areta,
Santiago Imberti and Mark Pearman
August 2025
Vote tracking chart: https://www.museum.lsu.edu/~Remsen/SACCPropChart1044+.htm
Comments from
Remsen: “NO. This research team is producing some great data on microevolution
among islands in this species. Fascinating
results that reveal important factors relative to the early stages of
differentiation. Thus, my “no” vote only
refers to the effort to apply taxonomy to this level of differentiation. Taxonomy is a totally artificial construct
devised by humans to provide labels for degrees of differentiation because we
have a tough time dealing with continuous variation. Using that scheme, these populations do not
fit our definition of species, and I would really have to work hard to accord
them subspecies (diagnosable unit) rank.”
Comments from
Areta: “NO. As
described in the proposal, the evidence is insufficient to consider subantarctica
as a different species, and I would be willing (in a positive spirit) to grant
it at most subspecific status.”
Comments from Zimmer: “NO. The Conclusion section
of the Proposal does an excellent job of summarizing all of the reasons why the
data provided by Rozzi et al. (2022a,b) should be considered insufficient for
the recognition of the Diego Ramirez population of A. spinicauda as a
distinct species. Furthermore, it’s not
clear to me that the Diego Ramirez population (differing only in morphometric
characters that could well be adaptive to extreme habitat/climate conditions
and the selective pressures they exert, and showing only minimal levels of
genetic differentiation in a single mtDNA marker) is truly diagnosable at any
taxonomic level, particularly given the noted failure to compare the morphology
and vocalizations of Diego Ramirez Aphrastura with the most proximate
Argentine populations that occur in the same habitat.”
Comments from Lane:
“NO. I believe the author team of the proposal make very good points against
the recognition of this taxon as a species.”
Comments from Stiles: “NO to considering Aphrastura subantarctica as a
separate species, based on its very short period of separation from spinicauda.
However, strong selection on morphology and behavior due to ecological factors
such as pronounced habitat differences (as in this case) can occur relatively
rapidly. Moreover, the rather small and apparently inconsistent differences in
vocalizations cast doubt on their possible functions as premating behavioral
isolating mechanisms.”
Comments from Claramunt: “NO. I agree with several points in
the proposal. The morphometric data does suggest that something is going on,
likely strong selection due to a very different habitat; but there is the
issues of lack of comparison with other populations that regularly use
tussocks. Microsatellites suggest genetic isolation, but, by their nature,
these may be showing genealogical closeness that does not reflect the degree or
relatedness more broadly. In particular, we don’t know if variation is nested
within the larger genealogical network of continental birds. Not an easy case,
but on the balance, I accept the recommendation of the proposal.”
Comments from Robbins: “NO, the authors of the proposal have provided
well-reasoned details on why these island populations don't merit species
status.”