Proposal (607.1) to South American Classification Committee
Results
of the voting on 607 (see below) were that 5 of 8 votes favored option 1, i.e.
no change in species limits but a transfer of one subspecies to another
species.
Therefore
607.1 becomes a YES/NO proposal as follows:
1. Retain three species of trumpeters, transferring ochroptera
from P. leucoptera to P. crepitans to avoid a paraphyletic P.
leucoptera.
(Van Remsen, 23 January 2015)
Comments from Remsen: “YES. This is the safest course given the lack of
direct studies of interactions at contact zones and lack of study of isolating
mechanisms. I am even a little reluctant
to make the subspecies transfer because this is based on an mtDNA gene
tree. I think Oppenheimer and Silveira,
and Ribas et al., have done a terrific job in setting the stage for more
focused and thorough sampling of populations in this group. The preliminary data from the headwaters all
point towards multiple species, and I look forward to one transect through one
of the potential contact zones for solid data on which to base a decision. Because Psophia
are of conservation concern, I think that this is a case in which a series of
photographs might be sufficient establish the pattern of plumage variation
across a transect. Pegan and Hruska did
a great job pulling these data together to make it easy for us or anyone else
to get a clear and quick grasp of the problem.”
Comments from Stiles: “YES.
I agree with Van on this one ... more
intensive genetic analyses and attention to vocalizations seem warranted. Incidentally, I find Bret’s observation of
singing by a trumpeter in captivity interesting – a source of vocal data? Has
anyone tried mirror-image stimulation with these birds? If males, it might work!”
Comments from Jaramillo: “NO – I am going to go contrarian on
this one, largely to state that there is something here in this paper that
would be good to acknowledge. I am not sure that the splitting up of Psophia will occur in this vote, but
keeping things as they are seems less palatable today to me, than beginning to
incorporate some of these findings.”
Comments
from Pacheco: “YES. In view of
insufficient sampling present in Ribas et al. and Oppenheimer/Silveira, it is
not appropriate changing boldly the arrangement of trumpeters.”
Comments
from Zimmer:
““YES. I think we have to do this given
the analysis of Ribas et al (2012), and given that the original Proposal 607 on
changing species limits in Psophia
failed to pass. However, like Alvaro, I
think there is more that we should have done with the original proposal
regarding species limits.”
Comments from Areta:
“YES.
This is the minimum necessary change to make our classification consistent with
phylogenetic data. Given insufficient sampling and meager biological data, this
is clearly a conservative change. As stated by others, the data presented by
Oppenheimer & Silveira (2009) and by Ribas et al. (2012) suggest that there
are more species-level entities in Psophia,
but critical information is needed before this move can be made with
confidence.”
=====================================================
Proposal (607) to South American Classification Committee
Recognize a
new species-level taxonomy of trumpeters (Psophiidae)
Effect on SACC: This
proposal reviews the taxonomy of the trumpeter family (Psophiidae). If passed, multiple new species would be
recognized: the revision we recommend would split Psophia viridis into
three species, P. viridis, P. dextralis, and P. obscura. Psophia crepitans napensis would be
elevated to species status as P. napensis, and P. leucoptera
ochroptera would be elevated to species status as P. ochroptera.
Background:
Psophiidae –
the trumpeters -- is a family in the Gruiformes currently consisting of three
species: P. leucoptera, P. crepitans, and P. viridis. New genetic and morphological evidence
suggests that this arrangement underestimates trumpeter diversity, as many (or
all) of the eight trumpeter taxa could be elevated from subspecific to species
status. Here we consider the evidence for how these eight taxa should be
classified.
Current
taxonomy recognizes the following taxa as species. The Gray-winged Trumpeter (P.
crepitans) occurs north of the Amazon River and is currently composed of two
subspecies, Psophia crepitans crepitans and P. crepitans napensis (hereafter
referred to as P. crepitans and P. napensis). The Pale-winged Trumpeter (P. leucoptera)
occurs south of the Amazon River and west of the Madeira River and includes two
subspecies, P. leucoptera leucoptera and P. leucoptera ochroptera (hereafter
referred to as P. leucoptera and P. ochroptera). The Dark-winged
Trumpeter (P. viridis) is endemic to Brazil and has three widely
recognized subspecies, P. v. viridis, P. v. dextralis, and P.
v obscura. A fourth subspecies, P.
v. interjecta, has generally not been considered valid. Hereafter these taxa will be referred to as P.
viridis, P. dextralis, P. obscura, and P. interjecta. The taxa within the P. viridis complex
were recently reviewed by Oppenheimer and Silveira (2009).
Trumpeters
are ground-dwelling birds that live in family groups that defend territories
together and breed cooperatively (Sherman 1996). They are poor fliers and prefer to walk
around obstacles, including water, rather than fly over them. They have been
reported flying over small streams and, in rare cases, swimming to escape
danger, but appear to seldom attempt to cross rivers. Hence, rivers are thought
to be an important barrier to trumpeter movement and therefore gene flow
between trumpeter populations. Trumpeter taxa are morphologically similar,
predominately distinguished by the color of their hind-wing patch (or mantle);
thus their English names, Gray-winged, Pale-winged and Dark-winged. This
hind-wing patch appears to be important in maintaining visual contact between
flock members and may play a role in displays (Sherman 1996).
Genetic
Data:
Ribas et al. (2012) sampled and sequenced
genetic mitochondrial genes (cyt b and ND2) from 62 individuals, including
representatives of all species and subspecies of Psophia.
Phylogenetic
Analysis:
Ribas et al.
used a Bayesian analysis to build a phylogenetic tree for all Psophia taxa
(Figure 1). This tree identified the eight trumpeter subspecies as monophyletic
entities, and found P. crepitans and P. leucoptera, as currently
defined, to be paraphyletic. Support values were high for branches separating
taxa, with the exception of weaker support for their node 5, which separates P.
crepitans and P. ochroptera. The Ribas phylogeny contains two main clades,
one corresponding to taxa north of the Amazon River and one corresponding to
taxa found south of the Amazon River. In
the Northern clade, P. napensis was found to be sister to P.
crepitans and P. ochroptera, while in the Southern Clade, P.
leucoptera was sister to the four subspecies currently subsumed within P.
viridis, with P. v. viridis sister to the remaining three taxa. In
addition, P. obscura was found to be sister to a clade comprised of P.
dextralis and P. interjecta (Ribas et al. 2012). This phylogeny
demonstrates clear differences between taxa but little to no structure within
them, indicating high gene flow within populations and lack of gene flow
between them. Psophia viridis
(i.e., the population formerly called P. viridis viridis) is somewhat of
an exception, showing higher nucleotide diversity and signs of structure in its
haplotype network; still, no evidence of hybridization with any other taxa was
found in this case.
Vocal Data:
There are no
vocal data studies that can inform this proposal. The three current trumpeter species seem to
produce similar vocalizations, but this has not been well studied.
Morphological
Data:
As
previously described, all trumpeters are somewhat similar in appearance,
differing mainly in the color of their wings (mantle) and lower throat (Sherman
1996).
Oppenheimer and
Silveira (2009) analyzed the P. viridis complex and found three
morphologically distinct groups defined by plumage color (based on a set color
key) and geography: the taxa are each unique to regions separated by large
rivers. Their proposed taxa are defined below:
1) P.
viridis: Found from the region between the Madeira and Tapajos rivers,
individuals have the distal and intermediate parts of the mantle Parrot Green,
with the proximal portion Dark Green. These individuals have a conspicuous
purple iridescence on the lower neck and wing.
2) P. dextralis:
Found between the Tapajos and Tocantins rivers, individuals have a mantle whose
distal portion is Olive Green, and whose intermediate and proximal portions are
Very Dark Brown. The lower neck and wing have either discrete or absent purple
iridescence.
3) P.
obscura: Found from eastern Tocantins to Maranhão, individuals had the
distal and intermediate portion of the mantle consistently Dark Green, with the
proximal portion Dark Brown. Their lower necks and wings had very much reduced
or no purple iridescence.
These
morphological differences, in conjunction with the absence of known contact and
hybridization zones, support the hypothesis that these taxa are genetically
isolated from one another and thus likely to retain their genetic and
phenotypic uniqueness in the future. In addition, this fact is supported by the
lack of clinal variation found in these morphometric parameters, both within
and across subspecies of Psophia (Oppenheimer and Silveira 2009). These
criteria coincide with those utilized by the British Ornithologists’ Union when
recognizing species based on morphology alone (Helbig et al. 2002). Oppenheimer
and Silveira found that P. interjecta was not unambiguously diagnosable
from P. dextralis based on plumage morphology so they recommend that it
be considered a subspecies of P. dextralis. However, genetic data (see
Genetic Data above; Ribas et al. 2012) shows evidence of structure between
these two populations, perhaps indicating an absence of gene flow between them.
Contact
zones:
These eight
taxa are allopatrically distributed and separated by large rivers, which likely
form isolating barriers to members of this ground-dwelling family. Nonetheless, the headwaters of these rivers,
where rivers are relatively narrow, may allow contact between different
populations.
Oppenheimer
and Silveira studied specimens from the headwaters of rivers that run between
the populations (where the rivers would serve as less effective barriers) and
found no evidence of clinal variation or intergradation in their mantle and
neck coloration. This observation suggests there is no gene flow between the
populations even where the rivers may serve as less effective barriers and the
populations may be in contact. Similarly,
Sherman (1996) reported that P. ochroptera may be in contact with P.
napensis at the western part of its range, but that there is no evidence
that interbreeding occurs there. Finally, Ribas et al. also studied specimens
collected from headwaters areas and found no mtDNA evidence for inter-taxon
hybridization in these areas. They also
found little geographic structure within populations, and that in some
instances specimens from headwaters had the same haplotypes as those of the
same populations from near the river mouths.
This indicates that there is little isolation within these taxa, but
strong isolation between them (see also Genetic Data section above).
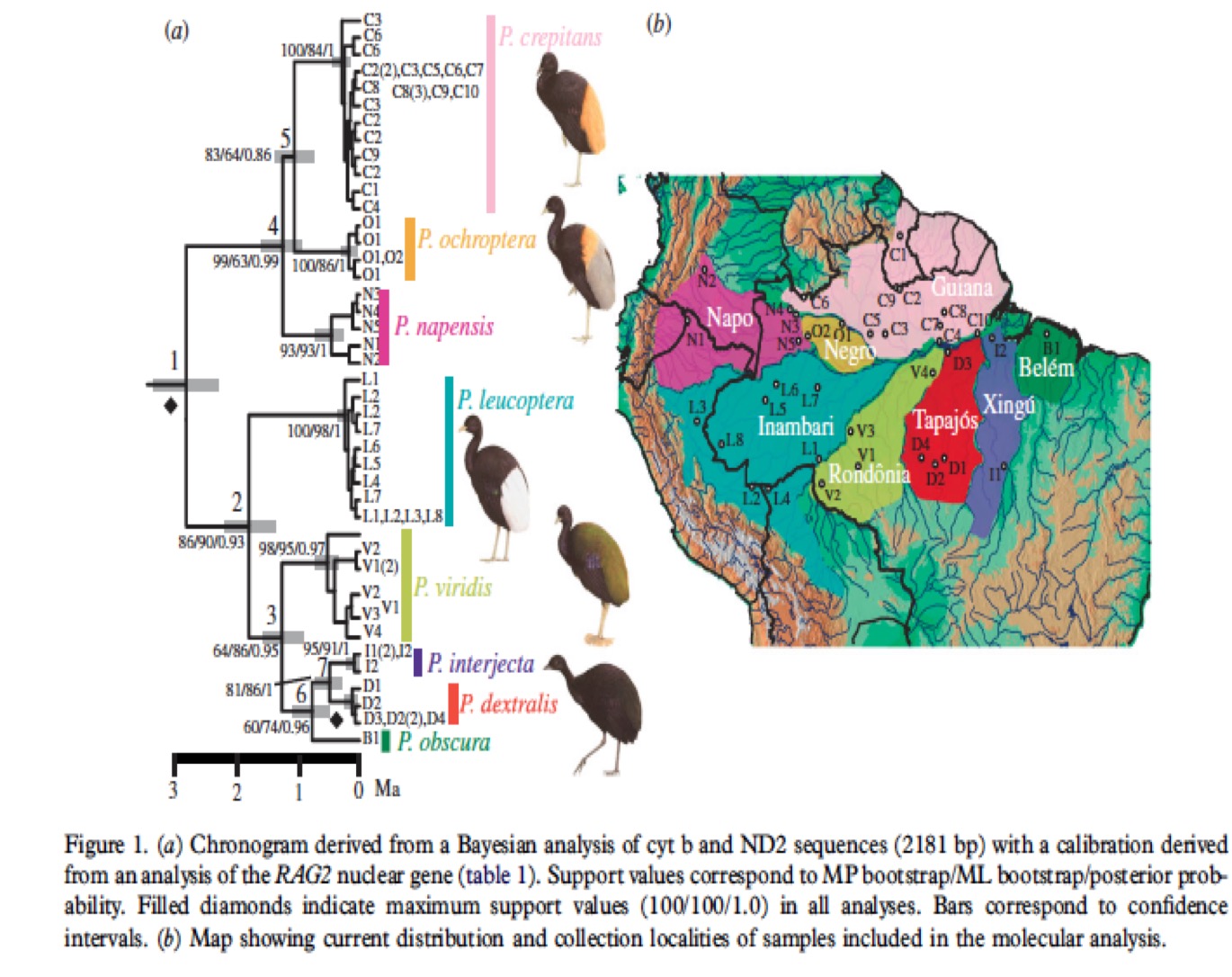
Conclusions:
This
proposal is largely based on the results of two studies. Oppenheimer and Silveira (2009) reviewed
morphological evidence surrounding species limits in Psophia viridis;
Ribas et al. (2012) used mitochondrial DNA to examine all three of the
current trumpeter species.
Taken in
conjunction with these birds’ natural history, the two studies complement each
other: Ribas et al. (2012) provides genetic support for the eight
trumpeter taxa and Oppeneheimer and Silveira (2009) shows that in the case of
the P. viridis complex, genetic differentiation is accompanied by
morphological divergence that suggests reproductive isolation and lack of
hybridization. We suggest that the morphological evidence presented in
Oppenheimer and Silveira (2009) can inform our inferences based on the Ribas
mtDNA phylogeny. Specifically, this bears on the question of whether distinct
taxa with rather shallow branch lengths ought to each be considered species or
whether subspecies affinities merely need to be rearranged. The fact that
Oppenheimer and Silveira’s morphological isolation data largely agrees with the
genetic conclusions of Ribas et al. suggests that P. viridis should be
split into multiple species; moreover, in this case they report no evidence of
clinal variation at headwaters, indicating that color, especially mantle color,
may be an important indicator of isolation.
This idea is further supported by the fact that the colored mantle
appears to be important in the social behavior of the species (Sherman 1996).
Given this
conclusion, it does not seem advisable to consider P. crepitans and P.
ochroptera as the same species given their readily diagnosable differences
in mantle coloration. Recognizing these
taxa as species also supports the recognition of P. napensis as a
species despite the fact that it is very similar in coloration to P.
crepitans; to do otherwise would create a paraphyletic species.
The one area
where morphological and genetic taxonomic recommendations differ is their
treatment of P. interjecta. This
proposed subspecies is usually not recognized and has been thought to be an
intergradation between P. dextralis and P. obscura (Sherman
1996). While Oppenheimer and Silveira
find it too similar to P. dextralis in plumage morphology to be
diagnosable as a species, Ribas et al. find genetic support for its distinction
as a taxon. This further strengthens the idea that no clinal variation exists
between trumpeter populations separated by large rivers; if P. interjecta
is a valid taxon and not an intergradation, no examples of this sort of clinal
variation exist. Ribas et al. found little to no mtDNA structure within each of
the eight taxa but strong structure between them, and use P. interjecta and
P. dextralis as particular examples of this result. However, given the
lack of morphological evidence, the relatively few samples of this taxon used
in the genetic study, and the shallow branch lengths of these taxa’s node on
the tree shown in Figure 1, it is less clear whether this taxon ought to be
elevated to a species or whether it should be recognized as a subspecies of P.
dextralis.
English names: Should this
proposal be passed, we leave it up to the SACC to determine English names of
the resulting species.
Taxonomic
possibilities:
There are quite a few permutations
of taxonomic possibilities because of the number of taxa involved, but we have
identified three possibilities that appear most reasonable given the new
genetic and morphological data:
1. Retain three species of trumpeters, transferring ochroptera
from P. leucoptera to P. crepitans to avoid a paraphyletic P.
leucoptera.
2. Recognize
seven species of trumpeters; recognize P. napensis and P. crepitans
and elevate all P. viridis subspecies to the species level except for P.
interjecta, based on lack of morphological evidence for this latter taxon’s
distinctness from P. dextralis; classify interjecta as a
subspecies of P. dextralis.
3. Recognize
eight species of trumpeters; all those described in the above option, and
additionally recognize P. interjecta based on lack of evidence of
structure within the taxon (contrasting with its genetic separation from P.
dextralis) and its geographic separation from P. dextralis by
a large river.
Recommendation:
We suggest that recent morphological
and genetic evidence is sufficient to warrant a novel species-level phylogeny
for trumpeters. As described above, we consider the evidence sufficiently
strong to support breaking trumpeters into multiple species instead of
rearranging subspecies into the currently recognized species. We therefore
recommend a “No” vote on Option 1. Instead, available evidence supports
recognizing at least seven species of trumpeters, and we therefore recommend a
“Yes” vote on Option 2. We are agnostic on the question of whether P.
interjecta ought to be elevated to full species status, but suggest that
this approach (Option 3) also a valid possibility.
Literature
Cited:
Helbig, A.J., Knox, A.G., Parkin, D.T., Sangster, G., & Collinson,
M. 2002. Guidelines for assigning species rank. Ibis, 144(3), 518-525.
Oppenheimer, M., & Silveira, L. F. 2009. A taxonomic review of the
dark-winged trumpeter Psophia viridis (Aves: Gruiformes: Psophiidae). Papeis Avulsos De Zoologia, 49(41), 547-555.
Ribas, C.C., Aleixo, A., Nogueira, A.C.R., Miyaki, C.Y., & Cracraft,
J. 2012.
A palaeobiogeographic model for biotic diversification within Amazonia
over the past three million years. Proc.
R. Soc. B 279, 681-689.
Sherman, P.T. 1996. Family Psophiidae (trumpeters). Pp. 96-107 in: del Hoyo, J., Elliott, A.,
& Sargatal, J. eds. (1996). Handbook of Birds of the World. Vol. 3.
Hoatzins to Auks. Lynx Edicions,
Barcelona.
Teresa Pegan and Jack Hruska, December 2013
========================================================
Comments from Stiles: “YES to proposal
1. The splitting of P. viridis into
multiple species seems a bit premature, as the genetic distances are not all
that great. Moreover, trumpeters are very vocal birds that spend most of their
time in dark forest understory; hence the importance of mantle color as an
isolating mechanism (vs. vocalizations) may be uncertain; here I think that
voice might be more important, and that some sonograms (hopefully with at least
some playback experiments) could help to shift the balance toward splitting up
the viridis complex, given that all
forms are apparently allopatric.”
Comments from Nores: “YES, to proposal 2. However, I agree
with Gary that voice might be very important, and that some sonograms and some
playback experiments could help especially in the viridis complex.”
Comments
from Zimmer: “YES. I’m conflicted on the specifics. I think that Proposal 1 is the minimum that
needs to be done – the genetic data are clear for removing ochroptera from leucoptera. I agree with Gary that vocalizations seem
likely to be of more importance than slight differences in mantle color. Unfortunately, I’m guessing that it is going
to be a long time before anyone comes up with meaningful sample sizes of
auditory recordings of each of these taxa.
As we have already seen with attempts to evaluate geographic differences
in vocalizations in Ortalis, it can
be very difficult to assess vocal differences in Psophia due to variation from one recording to the next in the
number of individuals vocalizing (and this usually being unknown). Also, I can’t help but think that the plumage
differences between ochroptera and crepitans/napensis have to be important when it comes to signal recognition,
mate choice, etc. – unlike the relatively
conservative plumage distinctions between the various taxa in the viridis complex, ochroptera versus anything else is pretty striking. If we treat ochroptera as a different species from crepitans, then we also have to split crepitans and napensis to
avoid a paraphyletic situation within crepitans. If we split those two taxa, then that opens
the door for recognizing the various populations of viridis as specifically distinct, an outcome that may be the
correct way to go, but one for which I am less enthusiastic (lacking vocal
data) than I am about splitting ochroptera
and crepitans. Despite the short branch lengths, I do think
that all of these taxa are likely on independent evolutionary trajectories,
especially given the seeming effectiveness of the river barriers in restricting
any potential for gene flow. I guess I
would weakly favor Option 2, to recognize 7 species (leucoptera, ochroptera, crepitans, napensis, viridis, obscura and dextralis). I can’t make myself go the extra mile in
splitting interjecta, which, from
what I can tell, isn’t even necessarily morphologically diagnosable.”
Comments from Robbins: “After reading the
proposal and Kevin’s comments, I support proposal 2, in recognizing seven
species of Psophia. Certainly, at a
minimum, proposal one should pass as the genetic data demonstrate that ochroptera must be transferred to crepitans if one is going to take the
narrow view that only three species should be recognized. Given the relatively little genetic
differentiation I appreciate how others might want to be conservative and
recognize only three species. Kevin is
correct that it may be a long time before pertinent vocal and display data are
obtained for these taxa. From what we
currently know, it does seem that the rivers, including the headwaters, are
acting as barriers to gene flow. Until
there are data to indicate otherwise, I lean more toward recognizing the seven
taxa outlined in proposal 2 as species.
Comments from Bret Whitney: “I see Ribas
et al, like most phylogeographic studies to date, as suffering from poor
sampling, especially for the two taxa east of the Xingu (= 2 for interjecta
and 1 for obscura), and uneven application of “results”. The
proposal says that Ribas et al. recovered “high support values for branches separating
taxa” except for “weaker" support value for crepitans and ochroptera.
I’d say several others are no or little better, especially where sampling
was poor (as it was for ochroptera = 2). Ribas et al (and,
implicitly, the proposal) don’t seem to have any reservations about
calling viridis, dextralis, and obscura monophyletic
species despite poor support, but do not mention similar, possibly
geographically linked structure within napensis (perhaps across the
Napo, a river known to separate a number of species-level taxa). Had
someone given a name to birds up in nw. Amazonas, Brazil, I imagine the
proposal would be recommending species-status for that, too. The
proposal, however, echoes a statement from Ribas et al to the effect of “the
phylogeny demonstrates clear differences between taxa butt little to no
structure within them…” The napensis example negates this assertion
and I don’t think they have enough of a sample of any taxa to make this
statement. Headwaters regions were woefully unsampled. Sampling was a little better in the
morphological study of Oppenheimer and Silveira, but it was also not robust.
“Another observation I have is two groups of ochroptera of
4-10 birds each that I have seen in Jaú National Park and near Barcelos that
had some individuals decidedly more orangish than others. This made me
wonder if the orange tint in the backs and remiges of these birds is real (=
genetically determined), or possibly an artifact of bathing constantly in
blackwater… something to at least think about, I suggest, in the same vein as
White-faced Whistling-Ducks showing variably orangish faces (but rarely
“white”) due to feeding habits. I can’t say I see orangish herons or egrets,
for example, more frequently in the Negro basin, but it does seem plausible
that pale-backed trumpeters bathing in dark-blackwater streams inside forest
could become heavily stained — and perhaps that is inevitable and to be
expected. Specimens of ochroptera are rare, and there are not many
available for analysis (Blake did have four males and a female and didn’t
mention any variation, but I would not expect him to unless it was dramatic).
“I would vote to accept proposal #1 and part of
#2, with napensis and ochroptera as subspecies of crepitans
(with a caveat on ochroptera as a valid taxon [tentatively
maintain it for now]); a monotypic leucoptera; and with the
split of viridis, dextralis, and obscura as species
because the analyses of Oppenheimer and Silveira, and Ribas et al., despite
very weak sampling, are congruent and mantle colors are quite different (in my
opinion); I think the split of the Dark-winged group represents an advancement
from where we are at present. I also have two recordings of adult,
captive obscura that are notably different from viridis and
dextralis, which are much more similar to each other — indicative
of differentiation, I would guess, but not enough on which to base judgments.
I would wait for more analysis of better sampling — morphological,
vocal, and genetic — of all taxa and putative populations before moving
for further revision; I do not see compelling reason(s) for doing more than
this at the moment. It certainly appears that interjecta is
individual variation, and we do not know whether overlooked cantatrix
Boeck 1884 from Beni represents an additional taxon or not; no sample from that
region was included.
“Some final thoughts — Would it not be possible to
include most or all of the specimens sampled by Oppenheimer and Silveira in
molecular analysis? It seems to me that most of them would have
sufficient material in their feet to permit extraction of tissue. I think
it would be especially wise to analyze all of the type specimens and a few
selected others, at least, to try to clear up some lingering doubts (that I
have, at least) about their provenance. Specifically,
Spix’s two leucoptera were labeled from the Rio Negro, but Hellmayr
decided they had to have come from south of the Amazon and west of the Madeira,
so fixed the type locality “left bank of the Rio Madeira”. He may be
correct in imagining that Spix obtained the specimens from villagers who had
them as pets, and they may well have originated west of the Madeira, but this
should be checked. Trumpeters are exceptionally fine pets and are traded
and kept by people all over the Amazon. I think it probable that the type
of P. viridis, from Parintins in the extreme lower part of the
Madeira-Tapajós interfluvium on the largest island of the Tupinambarana region,
was a pet that had been taken elsewhere; I doubt that trumpeters occur
naturally around Parintins. This should also be investigated genetically,
along with interviews of hunters and other locals on the island, who will
definitely know if trumpeters are in the forests there, or not.
Oppenheimer and Silveira cited a single specimen of viridis (MNRJ
9645) from “Machado, Mato Grosso”, an indeterminate locality.
It was the only example among their sample of 24 viridis that
had aberrant mantle coloration (two characters), and it was the only specimen
from Mato Grosso. They attributed the differences to individual variation
because another specimen “collected 70 km of this specimen (MZUSP 76728)
is typical…” Oppenheimer and Silveira did not indicate how they determined the
location of “Machado, MT” and did not map any Mato Grosso localities for viridis
(their figure 4). It would be desirable to analyze this interesting
specimen genetically in an attempt to understand something more about it.”
Comments
from Jaramillo: “YES on 1, and part of 2. Hmm, a problematic
one, but I find Bret’s comments valuable and convincing. Keep napensis and ochroptera as part of crepitans.
This moves us along, taking to consideration the new data we have, but
conservative on the parts that are still missing from this analysis.”
Comments
from Stotz: “YES to part
1. Currently I am unwilling to split up viridis based on current data.”
Comments from Remsen: “YES
on 1, following from Bret’s comments and waiting for additional data and a
proposal that would allow accepting of the part of 2 for which data are solid.”
Comments from Pérez-Emán: “YES
to Part 1. Nodal support grouping ochroptera
and crepitans has the weakest support
indicating that these three taxa are included in a polytomy. Besides, in the supplementary material you can
find the nuclear tree and there is a haplotype shared by both crepitans and napensis. This might be
ancestral polymorphism but it could also be gene flow. I think it is better to
be conservative until more data are available.”