Proposal (762) to South American Classification Committee
Treat Cranioleuca baroni and Cranioleuca antisiensis as conspecific
Effect on South American CL: This would lump two
taxa that are currently treated as separate species into a single species.
Background: Cranioleuca antisiensis was described by Sclater in (1858) from Cuenca, Azuay,
Ecuador and its subspecies palamblae
was described by Chapman (1923) from Palambla, Piura,
Peru. Cranioleuca baroni was
described by Salvin (1895) from central
Cajamarca, Peru (Huamachuco and Cajabamba). Later, Zimmer ( 1924) added Cranioleuca baroni capitalis from La
Quinua (near Cerro de Pasco), Pasco, Peru, followed by the description of Cranioleuca baroni zaratensis by Koepcke
(1961) from Bosque de Zarate,
Lima, Peru. Researchers have long recognized C. antisiensis and C. baroni
as being a species complex owing to similarities in plumage, vocalizations, and
habitat preferences, particularly where their distributional boundaries
apparently abut in central Cajamarca (Fjeldså & Krabbe 1990). All major taxonomic treatments of the group have
maintained them as separate species, but often with the caveat that they may
well be conspecific based on further collections and research (Remsen 2003). In 2002, Van Remsen
examined the series of C. antisiensis
and C. baroni at the Louisiana State
University Museum of Natural Science (LSUMNS), which hosts the largest series
of the complex, to provide a taxonomic recommendation to Tom Schulenberg for
the forthcoming field guide to the Birds of Peru. Two quotes from this
correspondence summarizing Van’s main conclusions are worth reiterating, “1. If
there is any way/reason to rank [C.] baroni
as a separate species, it is not evident from our series -- I'm not sure where
to draw the ‘line’. 2. Almost every locality has its own distinct
phenotype…”. Based on this, Schulenberg lumped C. baroni and C. antisiensis
in the Birds of Peru (Schulenberg et al. 2007) as C. antisiensis.
New Information and Analysis: Seeholzer and
Brumfield (2017) examined
morphological, plumage, and genetic variation of C. antisiensis-baroni using a series collected in 2010 and 2011 of
172 individuals from 19 populations spanning the geographic and environmental
breadth of the complex (Figure 1). They found that body mass of C. antisiensis-baroni
increases clinally almost threefold from north to south (Figure 2a) with individual extremes from 11.5 g to 31.0 g. The
cline is remarkably smooth despite some outlier populations on the arid west
slope of the Andes in Depts. Ancash and Lima, which were smaller than expected
given their transect positions.
Clinal
variation in plumage coloration and patterning is also obvious in a visual
examination of the series (Figure 3). The southern populations are generally greyer with
higher-contrast underparts than the northern populations yet with a smooth
transition between the geographic extremes. Within this general trend, however,
there is considerable within and among population variation. Seeholzer and
Brumfield (2017) quantified plumage
coloration and indeed found a strong correlation between north-south transect
position and an individual’s plumage score (Figure 2b). However, this relationship was messier than for body
mass.
The song of C.
antisiensis-baroni, a staccato series of accelerating, descending notes,
also varies clinally. GFS examined Macaulay Library and Xeno-Canto songs from across
the complex. The songs are variable in length, speed, and acceleration, but
these characters do not show any clear geographic pattern. However, the peak
frequency (pitch) of the songs decreases from north to south clinally (Figure 4). Although there is no body mass data associated with
these vocal data, it is clear that larger birds in the south have lower peak
frequencies than smaller birds in the north, as predicted by the scaling of the
syrinx with body size (Ryan & Brenowitz 1985). Like body size and plumage,
there is no discrete break in this vocal character with which to diagnose C. antisiensis from C. baroni.
Genetic data further reinforce that there is no way to
differentiate C. antisiensis from C. baroni. Mitochondrial data suggest
the phenotypic cline of C.
antisiensis-baroni formed relatively recently, with a maximum sequence
divergence between the geographic extremes of 1.1% and a divergence date of
~460,000 years (Derryberry et al. 2011). Although there is variation at the mitochondrial locus
ND2 that forms three clades, these clades show little geographic cohesion, with
samples from regions normally considered to be C. baroni or C. antisiensis
clustering together and samples from the same locality found in different
clades (Seeholzer & Brumfield unpublished data). Still, Seeholzer and
Brumfield (2017) found a clear signal
of clinal geographic population structure among 5,154 single-nucleotide
polymorphisms (SNPs) distributed throughout the genome. A principal components
analysis of this SNP matrix revealed three spurs of continuous genetic
variation consistent with a demographic scenario of isolation-by-distance (Figure 1a). These spurs were geographically structured, and the
relative position of the individuals in PC space conformed to the spatial
distribution of their respective populations (Figure 1b). Analysis of the SNP data with the clustering algorithm
ADMIXTURE (analogous to STRUCTURE) corroborated this pattern of smooth
transitions between ancestral populations, as pattern consistent with
isolation-by-distance (Figure 1b).
Recommendation:
Although Seeholzer and Brumfield (2017) did not address
taxonomy in their study, the taxonomic implications were clear and corroborated
Remsen’s assessment. The variation in morphology, plumage, song, and genetics
exhibit clinal variation that bridge the phenotypic extremes that were
described as C. antisiensis and C. baroni. With no discontinuities in
any characters with which to distinguish C.
antisiensis from C. baroni, their
current taxonomic status as distinct species is not justified under any species
concept. We recommend that these species be lumped under C. antisiensis, the name with priority. It makes sense to retain
the English name, Line-cheeked Spinetail, for stability and because all
populations exhibit the eponymous auricular streaking. However, we would like
the committee to consider changing the English name to the scientifically
evocative Clinal Spinetail, highlighting the striking pattern of clinal geographic
variation exhibited by C. antisiensis.
Literature Cited:
Chapman FM (1923) Descriptions of proposed
new Formicariidae and Dendrocolaptidae. American Museum Novitates, 86,
1–20.
Derryberry EP, Claramunt S, Derryberry G et
al. (2011) Lineage diversification and morphological evolution in a
large-scale continental radiation: the Neotropical Ovenbirds and Woodcreepers
(Aves: Furnariidae). Evolution, 65, 2973–2986.
Fjeldså J, Krabbe N (1990) Birds of the
High Andes. Zoological Museum, University of Copenhagen and Apollo Books,
Svendborg, Denmark.
Koepcke M (1961) Las razas geográficas de Cranioleuca
antisiensis (Furnariidae, Aves), con la descripción de una nueva
subespecie. Publ. Mus. Hist. Nat. Javier Prado (Ser. A. Zool.), 20,
1–17.
Remsen J V (2003) Family Furnariidae
(ovenbirds). In: Handbook of the birds of the world (eds Hoyo J, Elliot
A, Christie DA), pp. 162–201. Lynx Editions, Barcelona.
Salvin O (1895) On birds collected in Peru
by Mr. O. T. Baron. Novitates Zoologicae, 2, 1–22.
Schulenberg TS, Stotz DF, Lane DF, O’neill
JP, Parker, Theodore A I (2007) Birds of Peru. Princton University
Press, Princeton, NJ.
Sclater PL (1858) List of Birds Collected
by Mr. Louis Fraser at Cuenca, Gualaquiza, and Zamora in the Republic of
Ecuador. Proc. Zool. Soc. London, 449–461.
Seeholzer GF, Brumfield RT (2017)
Isolation-by-distance, not incipient ecological speciation, explains genetic
differentiation in an Andean songbird (Aves: Furnariidae: Cranioleuca antisiensis, Line-cheeked Spinetail) despite near
three-fold body size change across an environmental gradient. Molecular
Ecology, 1–18.
Zimmer JT (1924) New birds from central
Peru. Field Museum of Natural History (Zoological Series), 12,
51–67.
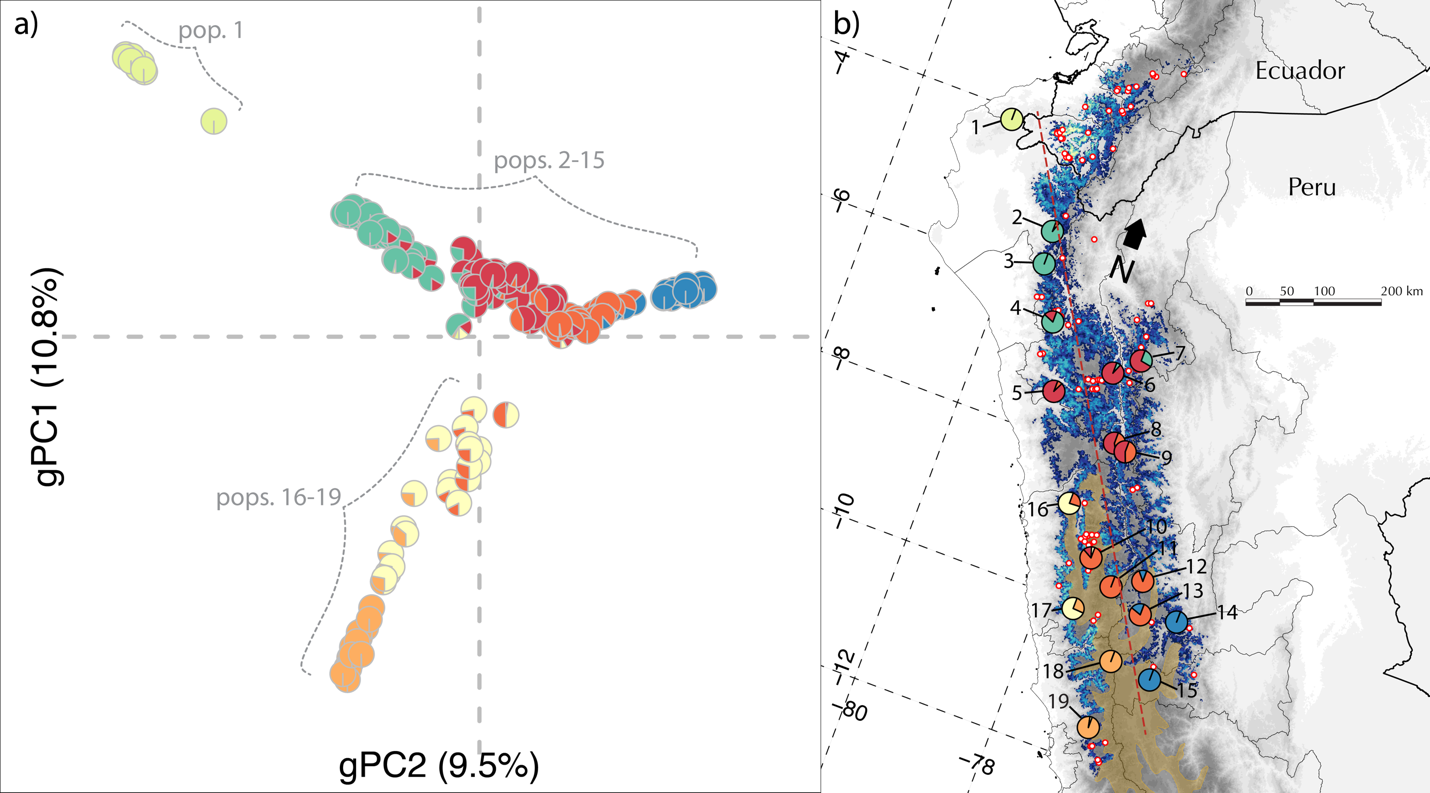
Figure 1. Genetic
variation and geographic distribution of C.
antisiensis. (a) The first two principal components of
the matrix of 5,154 SNPs across 172 individuals of C. antisiensis explained
20.3% of the genetic variance. Proportion of genetic variance explained by each
axis in parentheses. Pie charts represent the individual ADMIXTURE assignment
probabilities to ancestral populations at K = 7. (b) Species distribution model
of C. antisiensis. Pie charts are population averages of ADMIXTURE assignment
probabilities. Red dots with white centers are localities used in the MAXENT
model that did not have genetic data. Locality labels correspond to Table 1.
Red dashed line represents transect from orthogonal regression. The Central
Andean Wet Puna ecoregion (brown shading) forms a biogeographic barrier to
dispersal between the central Andean populations (10-15) and the SW slope
populations (16-19).
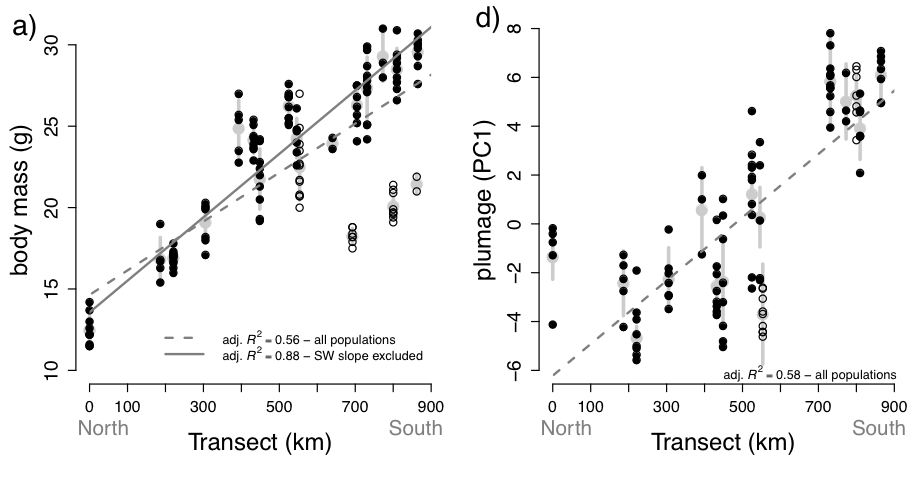
Figure 2. Clinal
variation in (a) body mass and (d) plumage color reproduced from Seeholzer and
Brumfield (2017) Figure 2a and 2d. Points represent individuals and are
overlain on the population means (larger grey circle) and standard deviations
(vertical grey bars). Individuals from the SW slope are represented as hollow
circles. The relationships for body mass were stronger when the SW slope
populations are excluded (solid line) than among all populations (dashed line).

Figure 3. (a) Representatives of the phenotypic cline
of Cranioleuca antisiensis-baroni
taken from points along the north (left) to south (right) transect. b) Side-by-side
comparison of the phenotypic extremes. Upper specimen from Cerros de Amotape,
Tumbes (pop. 1). Lower specimen from La Quinua, Pasco (pop. 15).
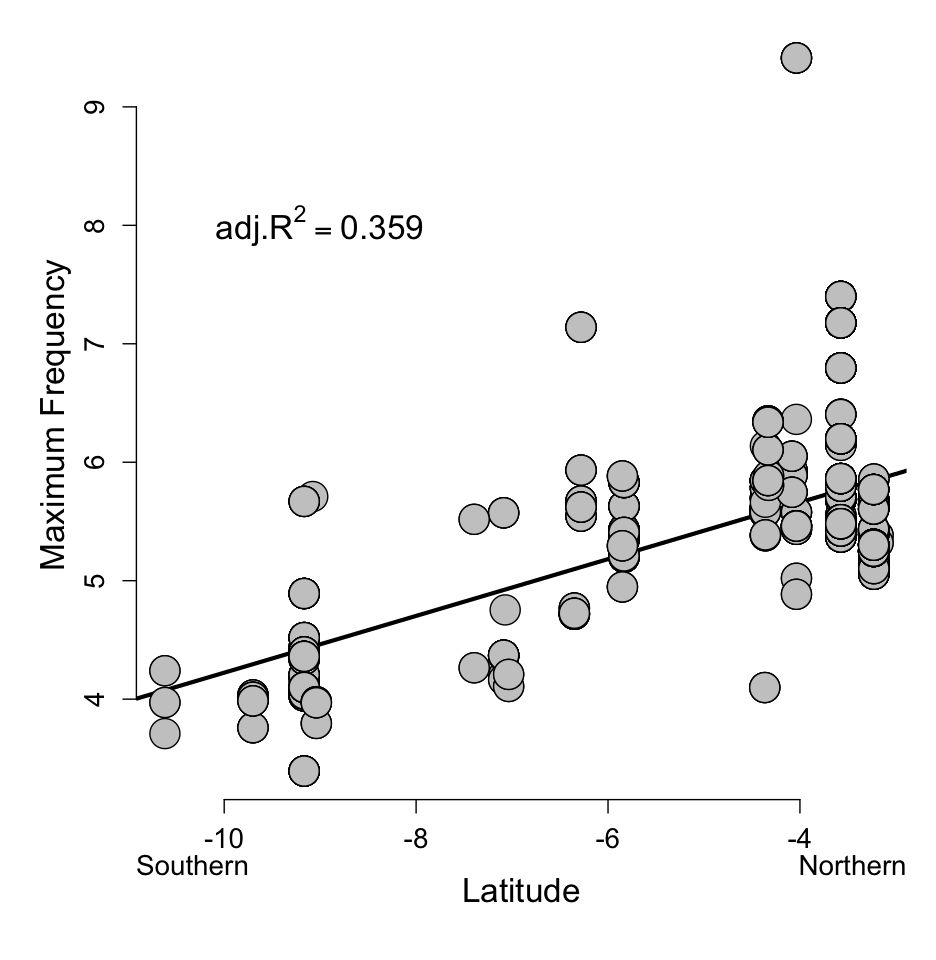
Figure
4. Clinal variation in the frequency (pitch) of C. antisiensis-baroni song (Seeholzer
unpublished data).
Glenn F. Seeholzer and Robb T. Brumfield, 19 December 2017
__________________________________________________________
Comments from Stiles: "YES. A thorough analysis of a curious
situation, and a good example of a study where genetic variation clearly trumps
morphological evidence for NOT splitting a species!"
Comments
from Areta:
"YES. A great quantitative example showing clinality in another Andean
furnariid."
Comments from Zimmer:
“YES. The thorough examination of
multiple data sets from the length and breadth of the geographic distribution
of the complex clearly establishes the clinal nature of the rather radical
morphological variation. Nicely done!”
Comments
from Remsen:
"YES. I was on Glenn’s committee and
know these data and this paper well. The
conclusion that a single species is involved is inescapable. To me, this is THE most spectacular example
of geographic variation in birds.”
Comments from Cadena: “YES. A beautiful example of
geographic variation and of the perils of using specimens from extremes of the
geographic distribution of taxa to make taxonomic inferences.”
Comments
from Jaramillo:
“YES – Wow! This is pretty amazing. Definitely the type of situation where a
thorough analysis, as was done, is the only way to arrive at a clear picture.”
Comments
from Pacheco:
“YES. A didactic case to always keep in mind: ‘With no discontinuities in any characters with which to distinguish C.
antisiensis from C. baroni’, their
current taxonomic status as distinct species is not justified under any species
concept.”