Proposal (784) to South American Classification Committee
Split Grallaricula leymebambae from G.
ferrugineipectus
This
proposal is based on excerpts from the following paper. Where mentioned, figure
and table numbers are kept the same as in the paper for ease of reference to
the publication.
Van Doren BM,
Freeman BG, Aristizábal N, Alvarez-R M, Perez-Emán J, Cuervo AM, Bravo GA.
2018. Species limits in the Rusty-breasted Antpitta (Grallaricula
ferrugineipectus) complex. Wilson Journal of Ornithology. doi:
10.1676/16-126.1.
Background
The
Rusty-breasted Antpitta (Grallaricula
ferrugineipectus) is a small antpitta in the family Grallariidae
distributed from Venezuela to Bolivia. Three subspecies are currently
recognized, each of which inhabits a distinct montane region: G. f. ferrugineipectus is found in
northern and western Venezuela and the Sierra Nevada de Santa Marta in adjacent
northern Colombia; G. f. rara in the
Eastern Andes of Colombia and the Sierra de Perijá, which straddles the
Colombia-Venezuela border; and G. f.
leymebambae in the Andean foothills from extreme southern Ecuador to
western Bolivia.
Current
knowledge of the distribution of the species has been improved by recent
discoveries of populations outside its traditionally known range. Although its
presence in Peru north of the Marañón River had been documented since the
mid-1950s based on 2 specimens taken independently by M. Koepcke and T. A.
Parker in Cancheque, Piura (Schulenberg and Parker
1981, Parker et al. 1985), there are now recent
records in the departments of Piura (Vellinga et al. 2004) and Lambayeque (Angulo Pratolongo et
al. 2012), as well as in the
Ecuadorian provinces of Loja and Pichincha (Athanas and Greenfield
2016; P. Coopmans, ornithologist, unpubl. data, collected 1994-2003). Likewise, only in the early 1980s was the species first
recorded in Bolivia (Schulenberg and Remsen
1982). More recently, MAR
and collaborators discovered a population in the Cauca Valley of the Central
Andes in the department of Caldas, Colombia that seems to be geographically
isolated from other conspecific populations. Taxonomic affinities of these
populations have never been formally assessed, and their taxonomic treatment
has been assumed to correspond to that of the geographically closest
populations.
Populations
differ somewhat in elevational distribution and habitat; subspecies rara and ferrugineipectus inhabit forested foothills from ~250 to 2200 m
asl. (Krabbe and Schulenberg
2003), and the Cauca Valley
antpittas are currently known only from one locality at 1000–1100 m asl.
Hereafter, these 3 populations will be referred to as the northern group. By
contrast, birds belonging to the southern group (populations from Ecuador,
Peru, and Bolivia) range substantially higher and inhabit montane forest from
1750 to 3350 m asl. (Ridgely and Tudor
2009). Southern group birds
seem closely tied to bamboo in the genus Chusquea
(Fjeldså and Krabbe
1990, Athanas and Greenfield 2016). This habitat association has not been documented for the
northern group, which can tolerate some degree of habitat degradation (Hilty and Brown 1986,
Niklison et al. 2008, N. Athanas, Tropical Birding Tours, 2017, pers. comm.).
The
3 subspecies of G. ferrugineipectus
were described based on differences in plumage and morphology. The subspecies
rara is the most divergent with respect to plumage, with a rich
rufous-brown underside and clear rufous tones on the crown and face, in
contrast to the dark brown to slate-brown upperside and rufous underside of
subspecies ferrugineipectus and leymebambae. These latter 2
subspecies both lack rufous on the
head, have duller underparts, and show an obvious white throat crescent; G. f. leymebambae differs from G. f. ferrugineipectus in larger size and darker overall coloration (Greeney 2013). Songs of the species also vary across its geographic
range. Most noticeably, G. f. leymebambae
gives slower and higher-pitched songs than G.
f. ferrugineipectus (Krabbe and Schulenberg
2003). The song of G. f. rara is poorly known and not
described in recent reference volumes (Krabbe and Schulenberg
2003, Greeney 2013). Finally, recordings
from northwest Ecuador demonstrate that this population’s songs seem slower
than those of other populations (e.g., see XC35333 on xeno-canto.org).
Because
populations of G. ferrugineipectus
are distributed allopatrically throughout the tropical mountains of northern
South America, reproductive isolation between subspecies cannot be directly
assessed. Early on, systematists proposed arrangements of species-level
taxonomy based on plumage differentiation within this complex. For example, G. f. rara was originally described as a
species because of its distinctive plumage (Hellmayr and Madarász
1914), while G. f. leymebambae was first described as
a subspecies (Carriker 1933). More recently, differences in distribution, morphology,
and vocalizations between G. f.
leymebambae and the northern group have led some authors (e.g., Ridgely and
Tudor 2009, BirdLife International 2017) to classify it as
a distinct species; however, no formal comparative analysis had systematically
examined genetic, vocal, and morphological variation within this complex.
New Information
Maximum-likelihood
and Bayesian analyses produced identical topologies supporting the
non-monophyly of Grallaricula
ferrugineipectus. Northern populations (i.e., G. f. ferrugineipectus, G. f.
rara, Cauca Valley, and Sierra Nevada de Santa Marta) form a strongly
supported clade that is sister to Andean populations of G. nana (albeit
with low support in the Bayesian species tree), whereas populations from Peru
and Bolivia (i.e., G. f. leymebambae)
are recovered as sister to G. lineifrons
(Fig. 2 and 3). The time-calibrated species tree estimated that the most recent
common ancestor between northern and southern groups split between 10.8 and
16.8 million years ago (mya; Fig. 3). Additionally, northern populations of G. ferrugineipectus exhibit some degree
of geographic structure and differentiation not entirely consistent with current
subspecific boundaries.
Northern
and southern groups significantly differed in the mean values of 13 of 15 vocal
traits; however, mean note maximum frequency was the only vocal character for
which 95% prediction intervals did not overlap (Figure 5; Table 1). To place
this result into context, we considered the number of vocal characters for
which 95% prediction intervals did not overlap between currently recognized Grallaricula species. The southern group
differed from G. nana in only one
vocal character (song pace), whereas populations in the northern group differed
from G. nana in 3 characters: mean
note maximum frequency, the frequency slope of the song, and the position of
the maximum frequency in the song. Both northern and southern groups differed
from G. lineifrons by several vocal
traits (7 and 4, respectively), and G.
nana differed from G. lineifrons
by 8 vocal traits (Table 1). Although northern and southern groups differed
significantly from one another in the mean value of all 6 morphological
characters, none was diagnosable at the 95% prediction level.
Discriminant function analysis
performed well at separating northern and southern groups based on both vocal
and morphological traits (Figure 7). For vocal traits, the cross-validated
correct classification rate was 100% for the northern group and 97.6% for the
southern group. The single discriminant factor loaded most heavily for mean
note maximum frequency. For morphological traits, the cross-validated correct
classification rate was 95.5% for the northern group and 100% for the southern
group; the discriminant factor was primarily composed of tarsus and tail
length.
Proposed Change
We found
that Grallaricula ferrugineipectus,
as currently recognized, is polyphyletic. The southern subspecies G. f. leymebambae is more closely related to G. lineifrons and G.
flavirostris than it is to the northern subspecies G. f. ferrugineipectus and G.
f. rara; in turn, these northern populations are more closely related to G. nana than to G. f. leymebambae. In
fact, the split between the northern and southern clades likely represents the
earliest divergence within the genus Grallaricula
(Fig. 2, GAB unpubl. data), and the age of this split is close to the start of
diversification of the Hylopezus-Myrmothera-Grallaricula
clade, estimated at ~13–21 mya (Ohlson et al. 2013). Hence, G.
ferrugineipectus as currently defined is polyphyletic and comprises
populations that belong to divergent and distinctive clades. We therefore
propose to elevate G. f. leymebambae
to species rank. We recommend the complex be considered to consist of 2 species
and, provisionally, 2 subspecies:
Species Grallaricula
ferrugineipectus (Sclater 1857)
Subspecies Grallaricula f. ferrugineipectus (Sclater 1857)
Subspecies Grallaricula f. rara Hellmayr and Madarász 1914
Species Grallaricula
leymebambae Carriker 1933
References
Angulo
Pratolongo, F., J. N. Flanagan, W.-P. Vellinga, and N. Durand. 2012. Notes on
the birds of Laquipampa Wildlife Refuge, Lambayeque, Peru. Bulletin of the
British Ornithologists' Club 132:162-174.
Athanas,
N., and P. J. Greenfield. 2016. Birds of Western Ecuador: A Photographic Guide.
Princeton University Press, Princeton.
BirdLife
International. 2017. Species factsheet: Grallaricula
leymebambae. Downloaded from http://www.birdlife.org/.
Carriker,
M. A. 1933. Descriptions of New Birds from Peru, with Notes on Other
Little-Known Species. Proceedings of the Academy of Natural Sciences of
Philadelphia 85:1-38.
Fjeldså,
J., and N. Krabbe. 1990. Birds of the high Andes: a manual to the birds of the
temperate zone of the Andes and Patagonia, South America. Zoological Museum,
University of Copenhagen, Copenhagen, Denmark.
Greeney,
H. F. 2013. Rusty-breasted Antpitta (Grallaricula
ferrugineipectus) in T. S.
Schulenberg, editor. Neotropical Birds Online. Cornell Lab of Ornithology,
Ithaca.
Hellmayr,
E., and J. Madarász. 1914. Description of a new Formicarian-bird from Columbia.
Annales Musei Nationalis Hungarici 12:88.
Hilty,
S. L., and W. L. Brown. 1986. A guide to the birds of Colombia. Princeton
University Press, Princeton, New Jersey.
Krabbe,
N. K., and T. S. Schulenberg. 2003. Rusty-breasted Antpitta (Grallaricula ferrugineipectus) in J. del Hoyo, A. Elliott, J. Sargatal,
D. A. Christie, and E. de Juana, editors. Handbook of the Birds of the World
Alive. Lynx Edicions, Barcelona.
Niklison,
A. M., J. I. Areta, R. A. Ruggera, K. L. Decker, C. Bosque, and T. E. Martin.
2008. Natural history and breeding biology of the Rusty-breasted Antpitta (Grallaricula ferrugineipectus). The
Wilson Journal of Ornithology 120:345-352.
Ohlson,
J. I., M. Irestedt, P. G. Ericson, and J. Fjeldså. 2013. Phylogeny and classification
of the New World suboscines (Aves, Passeriformes). Zootaxa 3613:1-35.
Parker,
T., T. S. Schulenberg, G. R. Graves, and M. J. Braun. 1985. The avifauna of the
Huancabamba region, northern Peru. Pages 169–197 in P. Buckley, M. Foster, E. Morton, R. Ridgely, and F. Buckley,
editors. Ornithological Monographs no. 36: Neotropical Ornithology. American
Ornithologists' Union, Washington, DC.
Ridgely,
R. S., and G. Tudor. 2009. Field guide to the songbirds of South America: the
passerines. University of Texas Press, Austin, Texas.
Schulenberg,
T., and J. Remsen. 1982. Eleven bird species new to Bolivia. Bulletin of the
British Ornithologists' Club 102:52-57.
Schulenberg,
T. S., and T. A. Parker. 1981. Status and distribution of some northwest
Peruvian birds. Condor 83:209-216.
Sclater,
P. L. 1857. Descriptions of Twelve New or Little‐known
Species of the South American Family Formicariidæ. Pages 129-133 in Proceedings of the zoological Society
of London. Wiley Online Library.
Vellinga,
W.-P., J. N. Flanagan, and T. R. Mark. 2004. New and interesting records of
birds from Ayabaca province, Piura, north-west Peru. Bulletin of the British
Ornithologists' Club 124:124-142.
Table
1. Vocal traits that
differ between northern and southern Rusty-breasted Antpittas and 2
congeners following the 95% prediction interval test. Numbers refer to the
following traits: (1) mean note peak frequency bandwidth (Hz); (2) mean note
bandwidth (Hz); (3) mean note maximum frequency (Hz); (4) duration of song (s);
(5) mean duration of each note (s); (6) rate of note delivery (notes per s);
(7) frequency slope of the song (Hz per note); (8) song peak frequency
bandwidth (Hz); (9) song bandwidth (Hz); and (10) position of the highest
frequency note.
|
|
G. ferrugineipectus (North) |
G. nana |
G. lineifrons |
|
G. ferrugineipectus (South) |
3 |
6 |
3, 7, 8, 9 |
|
G. ferrugineipectus (North) |
|
3, 7, 10 |
3, 4, 5, 6, 7, 8, 9 |
|
G. nana |
|
|
1, 2, 4, 5, 6, 7, 8, 10 |
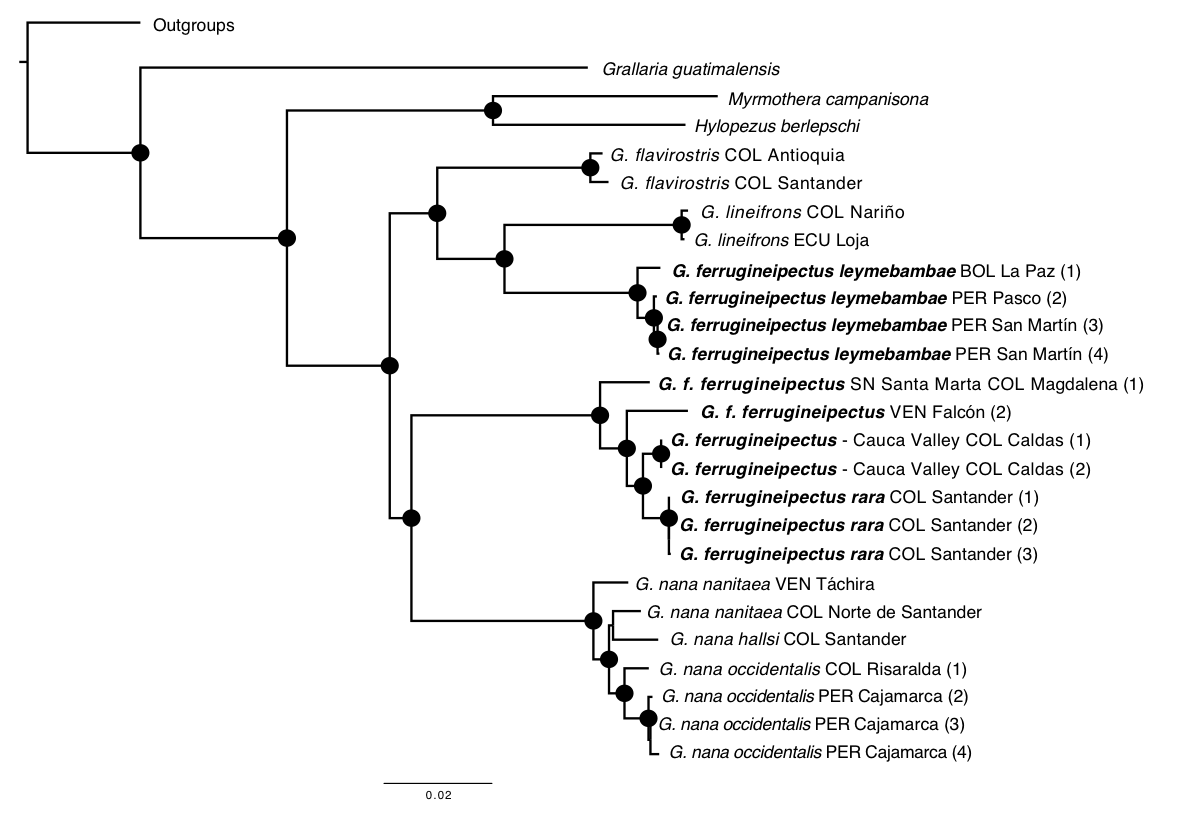
Figure 2. Maximum-likelihood phylogeny of a
subset of the Grallariidae. Note that Grallaricula ferrugineipectus sensu lato (in bold) is
polyphyletic. Black circles at nodes indicate bootstrap support values >70
based on 999 maximum-likelihood replicates.
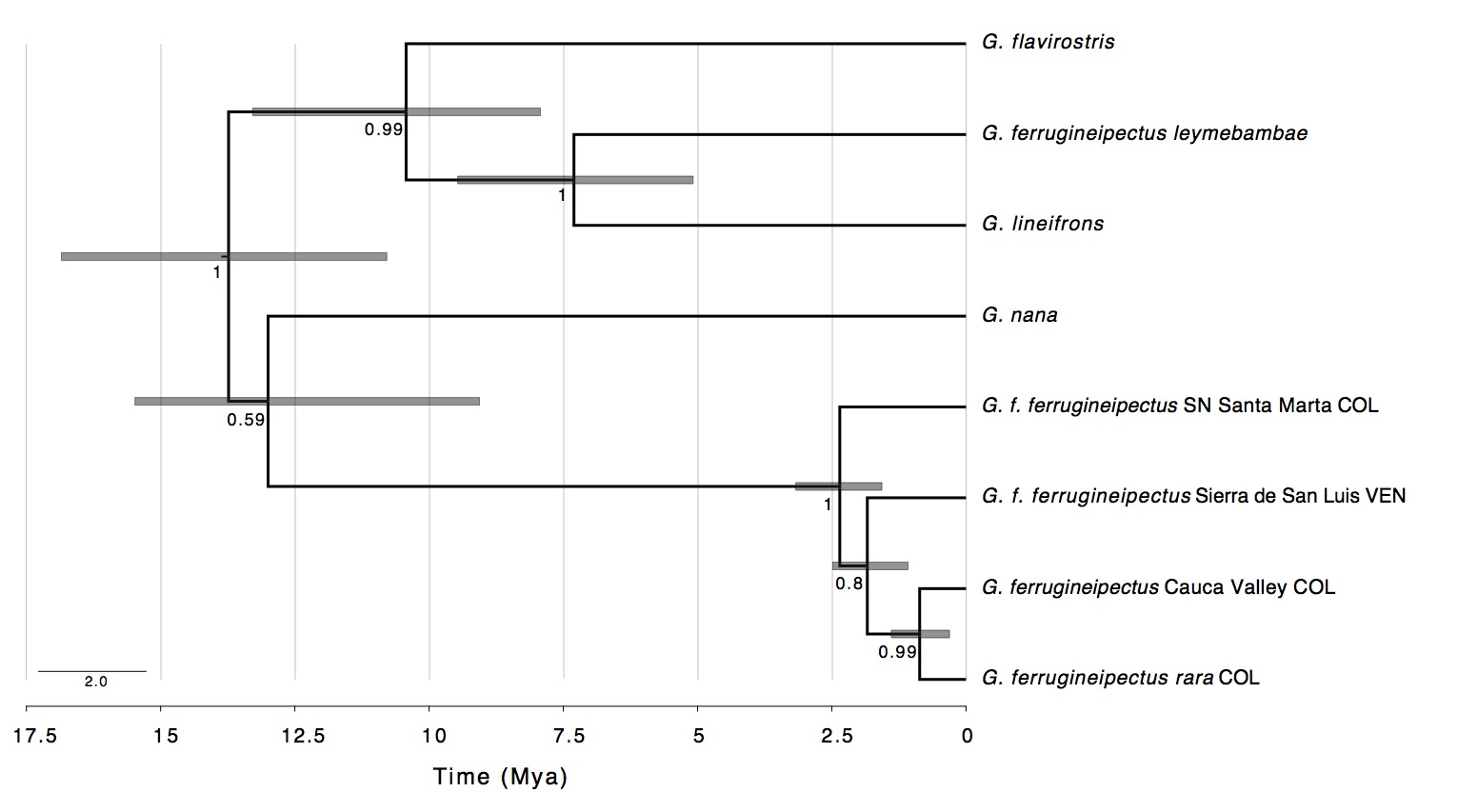
Figure 3. Bayesian
estimate of phylogenetic relationships and divergence times among a
subset of the Grallariidae. Grallaricula ferrugineipectus sensu lato is polyphyletic;
the northern and southern groups (G. f.
ferrugineipectus and G. f.
leymebambae) last shared a common
ancestor around 13 million years ago (mya). Bars at nodes indicate the 95% highest posterior density for the
inferred divergence time estimates. Numbers at nodes indicate posterior
probability support values.
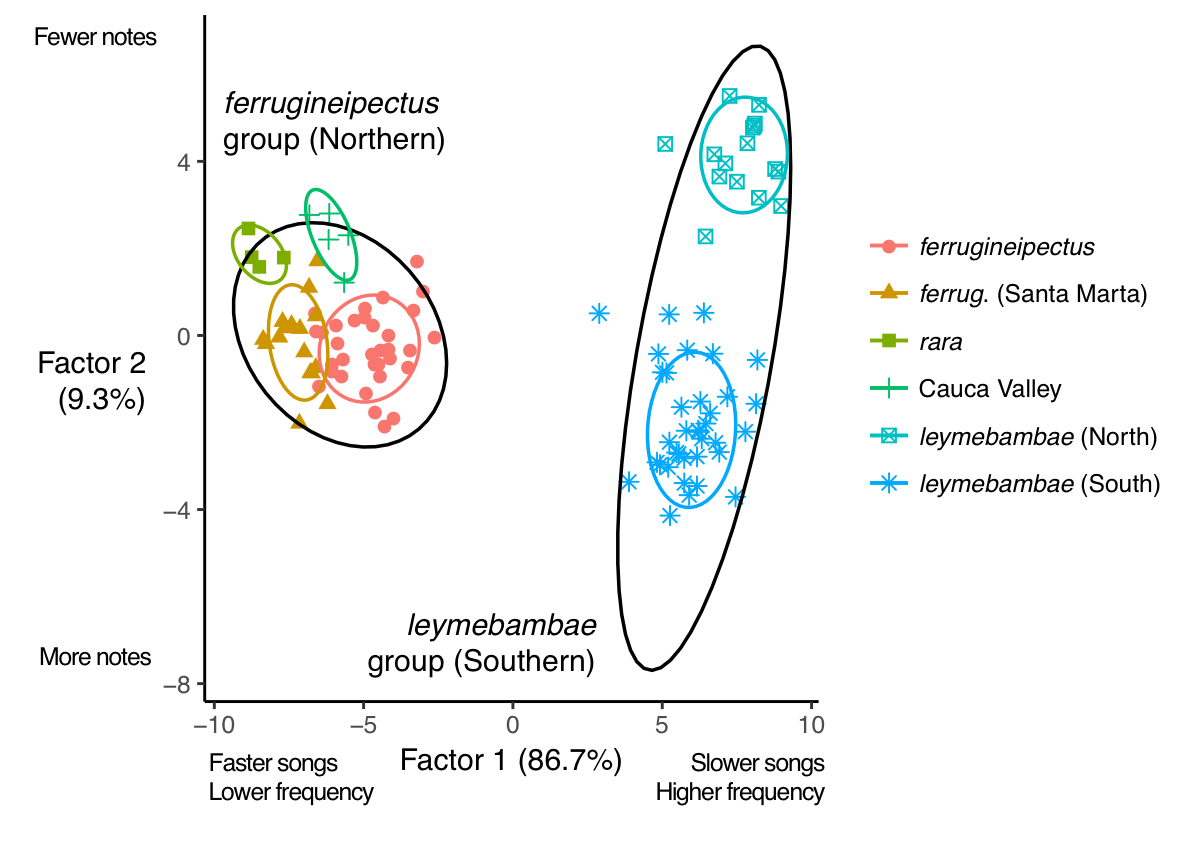
Figure 5. First 2 factors from discriminant
function analysis separating Rusty-breasted Antpitta subpopulations by
variation in 15 vocal traits. Black ellipses are 95% prediction ellipses for
northern and southern groups; colored ellipses are 75% prediction ellipses for
subspecies.
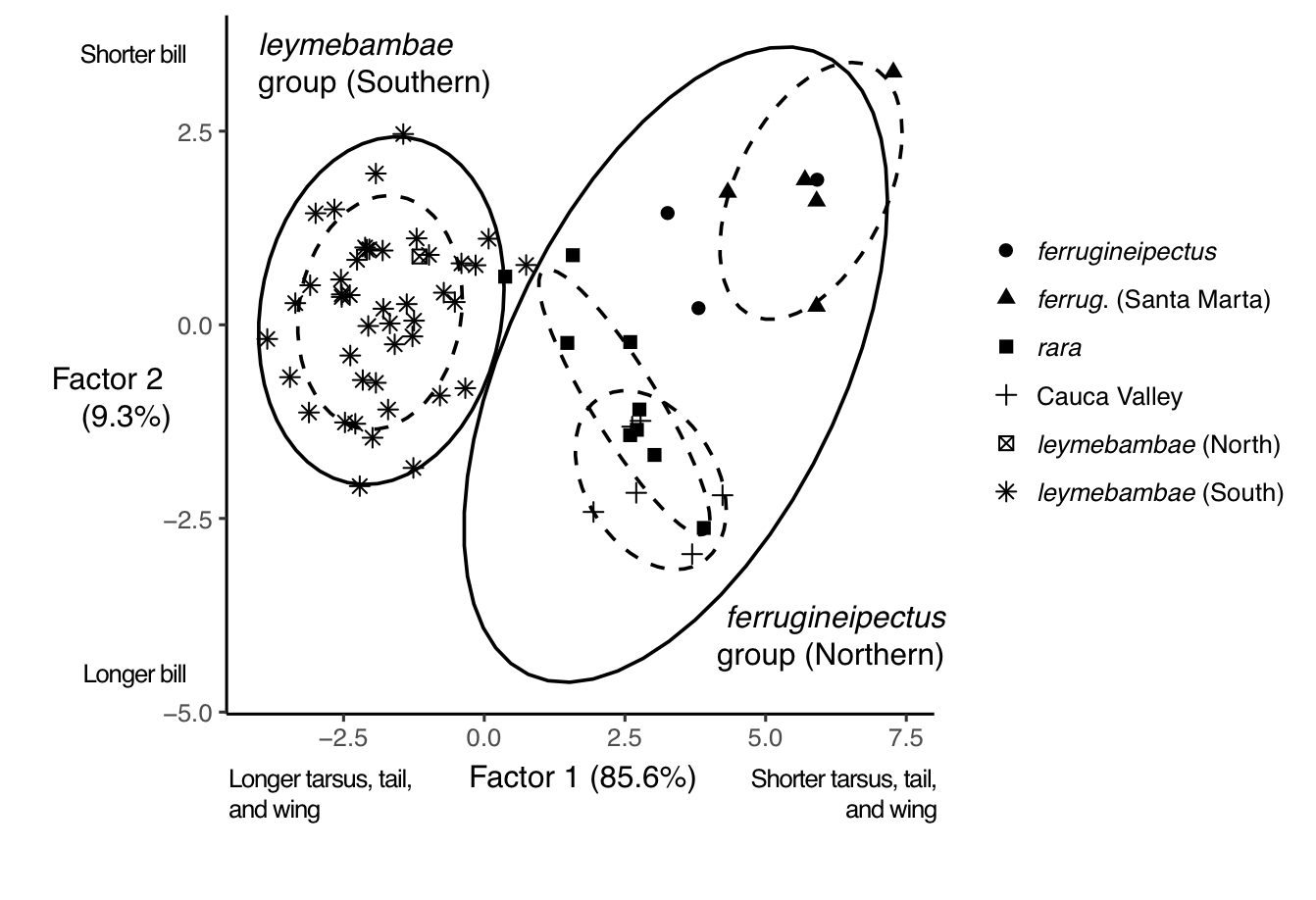
Figure 7. First 2 factors from discriminant
function analysis separating Rusty-breasted Antpitta subpopulations by
variation in 6 morphological traits. Black ellipses are 95% prediction ellipses
for northern and southern groups; colored ellipses are 75% prediction ellipses
for subpopulations.
Benjamin M. Van Doren, April 2018
__________________________________________________________
Comments from Remsen: “YES. Strongly
supported phylogenetic data make elevation of leymebambae to species rank essentially mandatory.
“We
need to think about English names (not mentioned in the paper). Because leymebambae was incorrectly included in Grallaricula
ferrugineipectus, this isn’t
really a split but an “extraction.” Therefore, I see no need to change the
English name of properly defined Grallaricula ferrugineipectus (Rusty-breasted
Antpitta) – it’s not really a parent-daughter thing in the phylogenetic sense.”
Comments from Robbins: “YES, phylogenetic
data clearly demonstrate that northern and southern ferrugineipectus are not sisters.
Although it is not too surprising that northern birds are more closely
related to nana than leymebambae, I am shocked that southern leymebambae is sister to lineifrons!”
Comments from Stiles: “YES. Clearly two
species are involved. Running through
the various “brown” names in Smithe, here are two
suggestions: Sepia-breasted (if the breast is really that much darker), or
Russet-breasted, which is a shade browner than “Rusty”. Confusingly similar? Perhaps,
but also recognizes the fact that the two are sufficiently similar to have been
considered conspecific ever since leymebambae was described.”
Comments
from Pacheco:
“YES. The evidence gathered clearly supports this proposition.”
Comments from Areta: “YES, a long overdue species treatment now
solidly substantiated with genetic, morphological and vocal data. It is finally
great to see these "enanos cachigordetes"
treated as separate species (as Paul Schwartz aptly called them a long time
ago).”
Comments
from Stotz:
“YES. Lots of data from a variety of
sources. On English names, I don't think I have any clever ideas what to
do. Best I can come up with is Ferruginous-breasted for the northern
group and Leymebamba for the southern group based on
scientific names. Leymebamba is a bad name,
obviously. Maybe Rufous-breasted?”