Proposal (871) to South American Classification Committee
Split
Great Thrush Turdus fuscater and
Chiguanco Thrush Turdus chiguanco
Background: The Chiguanco Thrush Turdus chiguanco has long been treated
as being comprised of 1-2 drab brown subspecies (the nominate and conradi) mainly on Andean slopes from
Ecuador through extreme northern Chile and Bolivia, and one more widespread
blackish race (anthracinus) from
central Bolivia east of the Andes through west-central Argentina and Chile
(e.g., Ripley 1964, Meyer de Schauensee 1970, Dickinson 2003, Dickinson and
Christidis 2014, Clements et al. 2019, Gill and Wright 2006). Both are common
and obvious in their ranges, and owing to their visually notable differences,
their conspecificity has been doubted (e.g., Sibley and Monroe 1993, Collar
2005), and Jaramillo (2003:230–231) mentioned that preliminary evidence
suggested vocal distinctions between the nominate group and anthracinus.
The Great Thrush Turdus fuscater, another common and conspicuous bird in the Andes
and outlying ranges from Venezuela to Bolivia, also has mostly drab brown
subspecies, with one (ockendeni) from
southeastern Peru being blackish. Hellmayr (1934) stated that T. f. fuscater differs from more
northern subspecies by its smaller size and especially weaker legs and feet,
blacker head, and stronger whitish chin spot, and Sibley and Monroe (1993)
listed the Bolivian nominate as a subspecies group possibly worthy of species
status, with all the other taxa subsumed under T. gigas Fraser, 1841. Collar (2005) suggested that T. ockendeni Hellmayr, 1906, may be best
treated as a separate species. Nevertheless, world checklists consistently
treat both of these taxa as subspecies of T.
fuscater (Dickinson 2003, Dickinson and Christidis 2014, del Hoyo and
Collar 2016, Clements et al. 2019, Gill et al. 2020).
The two species or species complexes are
broadly sympatric, and where this is the case, T. chiguanco is generally found in drier areas whereas T. fuscater is in more humid areas
(Ridgely and Tudor 1989, Ridgely and Greenfield 2001, Schulenberg et al. 2007).
Both occur even in towns and gardens and are among the most frequently seen and
most familiar birds in many areas (Clement 2000). Despite being sympatric over
huge areas through Ecuador and Peru, apparent intergradation between fuscater and chiguanco has been reported in Bolivia (or perhaps an undescribed
subspecies of T. chiguanco exists in
the La Paz and Cochabamba areas of Bolivia), suggesting that the blackish anthracinus may be better treated as a
race of the much larger, longer-tailed T.
fuscater (Fjeldså and Krabbe 1990) rather than of T. chiguanco.
New information:
Multiple phylogenies of the genus Turdus now published (Voelker et al.
2007, Nylander et al. 2008, Nagy et al. 2019) have only included one taxon each
of T. fuscater and T. chiguanco. In each of these studies,
the clade that included chiguanco and
fuscater also included T. fulviventris, T. olivater, T. nigriceps,
and T. serranus, but with varying
topologies within this clade. In Voelker et al. (2007), anthracinus is sister to serranus,
with fuscater gigantodes sister to
this clade. In Nylander et al. (2008), nominate fuscater is sister to a clade of nigriceps + serranus,
with nominate chiguanco sister to
this clade. In Nagy et al. (2019, entirely based on GenBank sequences), serranus + fuscater (race unstated as far as I could determine) are sister,
with nigriceps the next sister, then olivater, then chiguanco (race unstated).
In a newer phylogeny with much denser taxon
sampling and based on some 2000 loci of 53 species of Turdus, Batista et al. (2020) included T. reevei and, for the first time, two taxa each of T. fuscater and T. chiguanco: T. f. fuscater
(Bolivia); T. f. gigantodes (Peru); T. chiguanco conradi (Peru); and T. c. anthracinus. Each of these taxa
clustered in the clade including the same species listed in the preceding
paragraph, but the two forms of fuscater
appear to be non-sister (see Figure S9 of Batista et al. 2020, below). A sample
of T. f. fuscater (Bolivia) was
sister to a sample of T. olivater
kemptoni (Venezuela), and sister to this clade was T. olivater roraimae (tepuis). Although T. fuscater embedded within a clade comprising two subspecies of T. olivater may seem counterintuitive,
the characters given for nominate fuscater
by Hellmayr (1934) and the recent phylogenetic studies showing olivater to be a member of the same
clade suggest it may not be surprising after all. Nevertheless, the authors
indicate that further research is needed to validate this clade (Batista et al.
2020), and thus the non-monophyly of T.
fuscater may require corroboration.
T. fuscater gigantodes from Peru was sister
to T. chiguanco conradi from Peru,
and T. chiguanco anthracinus was
sister to this clade, the latter with an estimated divergence time from gigantodes + conradi of about 1 myr (Batista et al. 2020; see Fig. S9 below).
del Hoyo and Collar (2016) considered anthracinus
to be a full species, based on its blacker plumage, lack of streaked throat,
presence of narrow yellow eyering (absent or dark in chiguanco), and song with more but shorter and more rapidly
delivered whistled notes. Hellmayr (1934) also noted that anthracinus lacks the strong ochraceous or orange of the underwing
coverts and axillaries of chiguanco.
The two may be more or less parapatric in northern Chile (Jaramillo 2003) and
in northwestern Bolivia, judging from eBird data.
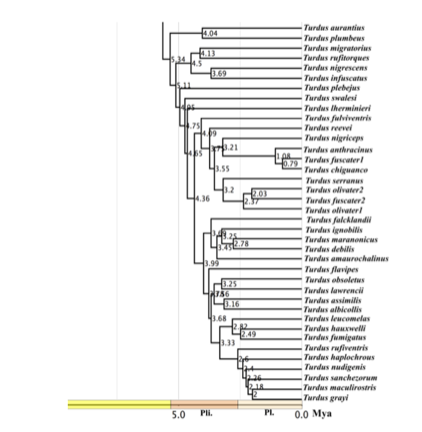
Figure
S9 from Batista et al. (2020).
The “leapfrog” pattern in plumage shown by T. f. ockendeni (not included in the
phylogeny) and its neighbors may be just that, or may suggest more than one
species is involved. In any case, as no phylogeny has as yet included ockendeni, even if nominate fuscater should be considered a separate
species from the gigas group, the
position of the distinctive ockendeni
is unclear.
Recommendation:
Given
the suggestion by Batista et al. (2020) that further work is necessary to
validate their finding that T. f.
fuscater is embedded within T.
olivater, and that even if non-monophyletic it isn’t yet clear which
subspecies would group together and how many species should be recognized, I
recommend voting NO at the present time on the motion to (A) split T. fuscater, pending further data and
analyses.
If
(A) passes, however, a separate proposal would be needed for English names.
The
case for specific status for T. chiguanco
anthracinus is stronger, however. In Batista et al. (2020), it is
genetically moderately divergent from the clade of T. f. gigantoides + T. c.
conradi, with striking color and eyering differences, and apparent song
differences. I do not think there is any convincing argument for retaining them
as conspecific. Another option would be to move anthracinus into T. fuscater,
but the question of intergradation in Bolivia is unclear, and the phylogeny in
Batista et al. (2020) does not support conspecificity. I recommend voting YES
on the motion to (B) remove anthracinus from
T. chiguanco, and (B1) consider anthracinus a separate species. (B2 would
be to move anthracinus into T. fuscater.)
If
(B1) passes, English names become an issue. Although Chiguanco Thrush has long
been used for the parent species including over much of Argentina, the name
mirrors the specific epithet of the nominate group, and good names for brown
thrushes are pretty much all taken. The name Chiguanco stems from a local
Aymara name (Jobling 2010), which makes it appropriate only for the nominate
and not anthracinus. I therefore
recommend a YES for (C), which would be to retain Chiguanco for T. chiguanco, as was done by del Hoyo
and Collar (2016).
For
T. anthracinus, del Hoyo and Collar
(2016) used Sombre Thrush, but it is in fact the male is a relatively handsome
bird compared to so many of its conspecifics. Hellmayr (1934) used Sooty Ouzel,
which conflicts with Sooty Thrush T.
nigrescens of Costa Rica and Panama. Coal-black Thrush, which mirrors the
scientific name, has been suggested (Ridgely and Tudor 2001), as has Dark
Thrush for the anthracinus group
(e.g., Sibley and Monroe 1993, Clements et al. 2019). But Pale Thrush Turdus pallidus is an East Asian
species, and a contrasting pair of names between non-sister taxa from different
hemispheres seems best avoided where there is a choice. Plus, Dark Thrush is
boring and non-specific. I recommend Coal-black Thrush, and if you agree, vote
YES for (D).
To summarize, here are the options:
(A)
Split
Turdus fuscater fuscater from T. gigas group
(B)
Remove
Turdus anthracinus from T. chiguanco
(B1) Consider T. anthracinus a distinct species
(B2) Consider anthracinus a subspecies of T. fuscater
(C)
Retain
Chiguanco Thrush for Turdus chiguanco
s.s.
(D)
Adopt
Coal-black Thrush for Turdus anthracinus
Literature Cited
Batista, R., U. Olsson,
T. Andermann, A. Aleixo, C. C. Ribas, and A. Antonelli (2020). Phylogenomics
and biogeography of the world’s thrushes (Aves, Turdus): new evidence for a more parsimonious evolutionary history.
Proceedings of the Royal Society B 287:20192400.
Clement, P. (2000).
Thrushes. Princeton University Press, Princeton, New Jersey.
Clements, J. F., T. S. Schulenberg, M. J. Iliff,
S. M. Billerman, T. A. Fredericks, B. L. Sullivan, and C. L. Wood (2019). The
eBird/Clements Checklist of Birds of the World: v2019. Downloaded from https://www.birds.cornell.edu/clementschecklist/download/
Collar, N. (2005).
Thrushes. In del Hoyo, J., A. Elliot, and D. A. Christie (Editors) Handbook of
the Birds of the World. Volume 10. Cuckoo-shrikes to Thrushes. Lynx Edicions,
Barcelona.
Collar, N., J. del Hoyo, E. de Juana, H. F. Greeney,
and G. M. Kirwan (2020). Chiguanco Thrush (Turdus chiguanco), version
1.0. In Birds of the World (S. M. Billerman, B. K. Keeney, P. G. Rodewald, and
T. S. Schulenberg, Editors). Cornell Lab of Ornithology, Ithaca, New York.
https://doi.org/10.2173/bow.chithr1.01
del Hoyo, J., and N. J.
Collar (2016). HBW and BirdLife International Illustrated Checklist of the
Birds of the World. Volume 2: Passerines. Lynx Edicions, Barcelona.
Dickinson, E. (Editor)
(2003). The Howard & Moore Complete Checklist of the Birds of the World. 3rd
Edition. Christopher Helm, London.
Dickinson, E. C., and
L. Christidis (Editors) (2014). The Howard and Moore Complete Checklist of the
Birds of the World. 4th edition. Volume Two. Passerines. Aves Press
Ltd., Eastbourne, UK.
Escobar Riomalo, M. P., E. Gongora, and S.
Arsitizabal Leost (2020). Great Thrush Turdus fuscater), version
1.0. In Birds of the World (T. S. Schulenberg, Editor). Cornell Lab of
Ornithology, Ithaca, New York, USA. https://doi.org/10.2173/bow.grethr1.01
Fjeldså, J., and N.
Krabbe (1990). Birds of the High Andes. Zoological Museum, University of
Copenhagen and Apollo Books, Svendborg, Denmark.
Gill, F. B., and M.
Wright (2006). Birds of the World: Recommended English Names. Princeton
University Press, Princeton, New Jersey.
Hellmayr, C. E. (1934).
Catalogue of Birds of the Americas and Adjacent Islands. Volume 13 Part 7.
Field Museum of Natural History, Chicago.
Jaramillo, A. (2003).
Birds of Chile. Princeton Field Guides, Princeton and Oxford.
Jobling, J. A. (2010).
The Helm Dictionary of Scientific Bird Names. Christopher Helm, London.
Meyer de Schauensee, R.
(1970). A Guide to the Birds of South America. Livingston, Narberth,
Pennsylvania.
Nagy, J., Z. Végvári,
and Z. Varga (2019). Phylogeny, migration and life history: filling the gaps in
the origin and biogeography of the Turdus
thrushes. Journal of Ornithology 160:529–543.
Nylander, J. A. A., U.
Olsson, P. Alström, and I. Sanmartín (2008). Accounting for phylogenetic
uncertainty in biogeography: a Bayesian approach to dispersal-vicariance
analysis of the thrushes (Aves: Turdus).
Systematic Biology 57:257–268.
Remsen, J. V., Jr., J.
I. Areta, C. D. Cadena, S. Claramunt, A. Jaramillo, J. F. Pacheco, J. Perez
Emán, M. B. Robbins, F. G. Stiles, D. F. Stotz, and K. J. Zimmer (Version 11
February 2020). A classification of the bird species of South America. American
Ornithological Society. http://www.museum.lsu.edu/~Remsen/SACCBaseline.htm
Ridgely, R. S., and G.
Tudor (1989). The Birds of South America. Volume 1. The Oscine Passerines.
University of Texas Press, Austin, Texas.
Ridgely, R. S., and P.
J. Greenfield (2001). The Birds of Ecuador. Volume 1. Status, Distribution, and
Taxonomy. Cornell University Press, Ithaca, New York.
Ripley, S. D. (1964).
Subfamily Turdinae, Thrushes. In Mayr, E. and R. A. Paynter (Editors)
Check-list of Birds of the World. Volume 10. Harvard University Press,
Cambridge, Massachusetts.
Schulenberg, T. S., D.
F. Storz, D. F. Lane, J. P. O’Neill, and T. A. Parker III (2007). Birds of
Peru. Princeton University Press, Princeton and Oxford.
Sibley, C. G., and B.
L. Monroe, Jr. (1993). A World Checklist of Birds. Yale University Press, New
Haven, Connecticut.
Voelker, G., S. Rohwer,
R. C. K. Bowie, and D. C. Outlaw (2007). Molecular systematics of a speciose,
cosmopolitan songbird genus: defining the limits of, and relationships among,
the Turdus thrushes. Molecular
Phylogenetics and Evolution 42:422–434.
Pamela
C. Rasmussen, July 2020
Comments
from Peter Boesman:
“The proposal briefly mentions ‘apparent song differences’
based on what can be read in del Hoyo and Collar (2016). I just would like to add that del Hoyo and
Collar (2016) mentioned vocal differences based on my brief vocal analysis. It was obviously not an in depth study, but
at least it reflects more in detail what I found by comparing available sound
recordings. See:
Boesman, P. (2016). Notes on the vocalizations of Chiguanco Thrush (Turdus chiguanco). HBW Alive Ornithological Note 307. In:
Handbook of the Birds of the World Alive. Lynx Edicions, Barcelona. https://doi.org/10.2173/bow-on.100307 https://static.birdsoftheworld.org/on307_chiguanco_thrush.pdf”
Comments
from Areta:
“I think much more information is needed here. I attach a segment of Figure S3
from Batista et al. (2020), which is better to understand the names of the taxa
sampled and their relationships. The proposal was difficult to read, because it
alternated between species-level and subspecies-level taxa, and because the
problems arise when looking at the subspecific-level, I´ve found it confusing
in several passages. I believe that a specific study taking care for the
several missing pieces of the puzzle is necessary before we can reach a solid
conclusion.
“I
agree in that at first sight, it is obvious that anthracinus is different from chiguanco
in plumage and general aspect. But when one digs further in, some interesting
problems arise.
“First,
geographic sampling is far from complete, and there are few samples per
species. Because the relationships are so poorly known, I wouldn´t be surprised
if other taxa fall in other parts of the tree. We lack samples of ockendeni, gigas, cacozelus, clarus, and quindio (there
might be some more ssp. of "fuscater"
around), so it would not be clear where to put all these taxa. What would be
the logic to place them in the fuscater
or gigas groups?
“Second,
nominate chiguanco has not been
sampled, only conradi. Interestingly,
conradi has been found to be sister
to gigantodes, in which males have a periocular but females do not (perhaps
this is the case in all the "gigas"
group), whereas both sexes of chiguanco
and conradi lack periocular ring, and
both sexes of anthracinus have it.
Note that the divergence between all these forms is quite recent, and although gigantodes and chiguanco conradi appear to coexist locally (suggesting that they
behave as good species?), we then will have to confront with nomenclatural
matters as to what to do with gigantodes
and allies in the "gigas"
group.
“Third,
the vocalizations of the whole group have not been adequately characterized.
Several song types alternate in anthracinus
(the taxon I am most familiar with), such that quick analyses might not be able
to characterize adequately the vocal gymnastics in this taxon. Also, we would
need to take a comparative analysis including fuscater and chiguanco
(and all the taxa currently included within them) in order to understand what
is going on.
“To
conclude, although evidence strongly suggests that multiple species are
currently included in both T. fuscater
and T. chiguanco as currently
circumscribed, the situation seems complex, and we lack enough data to make
informed decisions on several fronts. The problems are quite clear, and this is
what these studies have shown. However, they have not provided a solution. This
will need more data specifically attempting to solve the lingering species
limit uncertainties and the phylogenetic relationships of missing taxa. Until
then, sorting this out will necessarily involve a large amount of guesswork on
how to sort out the various ssp. to species.
“So
my votes are:
(A) Split Turdus fuscater fuscater
from T. gigas group --- NO. Not enough evidence. Also, what would we
do with ockendeni and the many other
ssp.? How can we be sure of where to put them? And what about T. olivater, which would have been rendered paraphyletic by the inclusion of fuscater as more closely related to ssp.
kemptoni than to roraimae? (and we do not know where true olivater falls, although it seems very likely that it will fall in
the same clade with kemptoni, roraimae, and fuscater). If we
split fuscater from gigas, then will we also split fuscater from its close sister olivater? and gigas as specifically distinct from its close sister chiguanco? If we do not split chiguanco from gigas, why would we then be splitting anthracinus from chiguanco[conradi]-gigas?
(B) Remove Turdus anthracinus from T. chiguanco
(B1) Consider T. anthracinus a distinct species --- NO. Not enough evidence.
(B2) Consider anthracinus a subspecies of T. fuscater --- NO. No evidence at all for this move, as the evidence is suggesting that
nominate fuscater belongs to the olivater group, and not to the "gigas-anthracinus" one.
“Figure S3 from Batista et al. 2020

Comments
from Stiles:
“NO to all. I agree with Nacho: there are too many gaps in the available data
to permit reaching a sensible decision. Too many subspecies lacking genetic
data, morphological information also incomplete or sketchy. Just from my
experience in Colombia, where there are three subspecies: gigas (Cordillera Oriental) –
huge, dull grayish-black, very long-tailed; quindío (Cordillera Central) –
smaller, more blackish, tail less long; cacozelus
(S. N. Sta. Marta) – large, more
brownish-black, tail fairly long. At least the first two have adult males with
yellow eyerings, lacking in females. In my experience, the song of gigas is extremely variable – and they
usually sing only before dawn. Disentangling this complex would make a nice
doctoral thesis; for the moment, best to stay with the (probably
unsatisfactory) status quo until a thorough study is available.”
Comments
from Claramunt:
“NO. The situation is very complex and further
studies are sorely needed. I don’t think that with the information at hand we
can make informed decisions. Relocating subspecies seems out of our current
tasks, as we don’t list subspecies.”
Comments
from Robbins:
“NO. Clearly much more information is needed before one can make an informed
decision about species limits within this complex.”
Comments
from Remsen:
“NO to all. This is a complex situation
that really needs a comprehensive analysis.
Batista et al. (2020) make it clear to that multiple that multiple
species are involved and have thus moved us forward considerably, but the taxonomic
details at this point are murky.”
Comments from Zimmer: “NO, for the reasons stated in the
recommendation in the Proposal, namely: 1)
Batista et al. (2020) concluded that more work was needed to confirm
that T. f. fuscater is embedded
within T. olivater; and, 2) Even if
this result is validated, it is not clear which subspecies would group
together, and how many species would need to be recognized. (B1) NO to considering anthracinus a distinct species.
There are too many gaps in what we know of the relationships of the many
subspecies in the species-complexes involved, and we don’t have anything more
than the barest of vocal analyses. I
would suggest that vocal analyses involving a complex, multi-taxon comparison
of an oscine passerine group, in which song dialects are likely to be modified
by learning (particularly one involving a group of accomplished vocalists such
as thrushes), would need to be particularly thorough and rigorous in order to
have much meaning. We clearly don’t have
anything approaching such rigor to assist in making the decisions we are being
asked to make at this time.”
Comments from Pacheco: “NO to all. We just
realized that there are many gaps to be filled, but nothing is particularly
safe at the moment for making substantial decisions.”
Comments from Lane: “NO. I agree with others that the
studies used in this proposal simply don't have the sampling necessary to make
the case for a split. These are family-wide phylogenetic papers that did not
conduct proper sampling to tackle a question such as this one, much as in
previous proposals such as those on Cacicus species limits.”
Comments
from Jaramillo:
“A
– NO. This may end up being the correct course of action, but more taxon
sampling is needed.
“B1
– YES. I realize I am the only one. Yet we have a distinctive taxon, genetic
information, they are nearly parapatric without sign of intermediates, a
terminal (southernmost) distribution. In essence to me this is the most clear
cut of what will likely be many future changes here once the taxa are sampled
more densely.
“B2
– NO.”
Comments
from Bonaccorso:
“NO to all. Since this is such a complex system, with
so much (apparently homoplastic) plumage similarity, complete sampling will be
needed to make a decision. Such an analysis should include all species and
subspecies (if possible). Also, as in many other cases, it would be important
to have systematic and explicit treatments of morphological and song
differences, and a clear account of geographic distributions, highlighting and
areas of sympatry and allopatry.”