Proposal (881) to South
American Classification Committee
Treat Lepidocolaptes
falcinellus as a subspecies of L. squamatus
The current SACC notes reads:
"138.
Lepidocolaptes falcinellus was formerly (e.g., Pinto 1937, Peters
1951, Meyer de Schauensee 1970, Sibley & Monroe 1990, Ridgely & Tudor
1994) considered a subspecies of L. squamatus, but Silva &
Straube (1996) provided evidence for why it should be treated as a species, and
this was followed by Marantz et al. (2003)."
However, the information to justify the treatment of falcinellus as
a separate species from squamatus is weak, and a return to the
previous classification seems warranted.
Silva & Straube (1996) analyzed 217 specimens of wagleri, squamatus,
and falcinellus. In doing so, they (1) obtained measurements (which were
not very informative, and are not discussed any further here) and (2) defined discrete
character types for three body parts which form the bulk of their
arguments.
We synthesize the data relevant to this proposal in the following
summary table.
Summary table
|
|
Head |
Back |
Tail |
Voice (call) |
|
wagleri |
1 |
1 |
1 |
|
|
Río São Francisco |
||||
|
squamatus |
2 |
2 |
1 |
|
|
Río Paraíba/ 22°S |
||||
|
falcinellus |
3 |
3 |
2 |
|
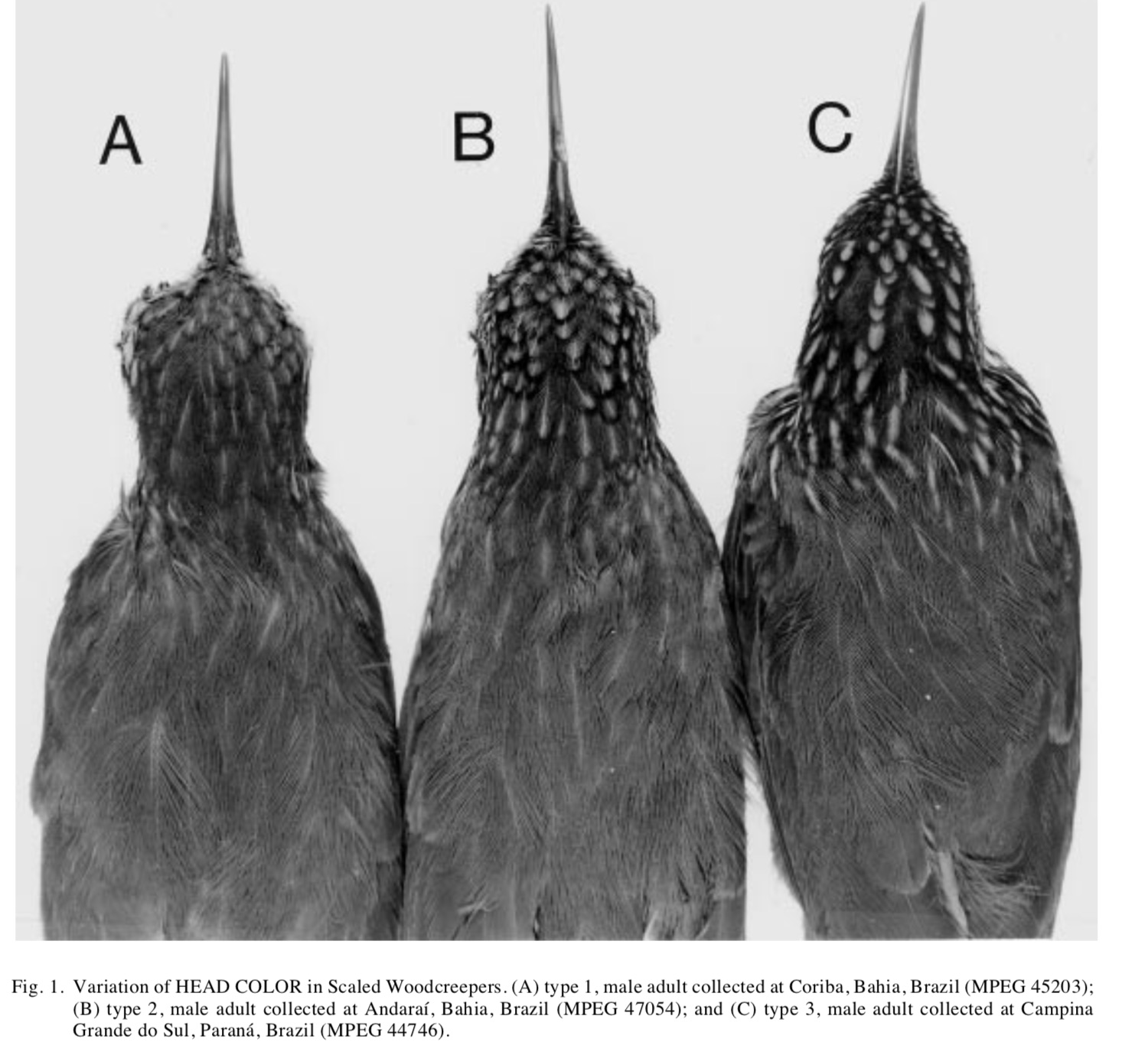
HEAD: 1 = brown
feathers with pale shaft lines; 2 = brown feathers with a small buff or whitish
spot, frequently edged with black or dusky at the tip; 3 = black feathers with
broad buff stripes (see Fig. 1)
BACK: 1 =
Sanford’s Brown × Burnt
Sienna; 2 = Tawny × Ochraceous
Tawny; 3 = Antique Brown
TAIL: 1 = Burnt
Sienna; 2 = Chestnut
The main arguments by Silva &
Straube (1996) are summarized in these paragraphs (see also our Summary
table):
"The three
allopatric populations of Scaled Woodcreeper (Fig. 4) may be separated from one
another with 100% confidence by using a combination of two discrete plumage
characters (HEAD and BACK). The third character (TAIL COLOR) separates the
three populations into only two groups: wagleri plus squamatus and
falcinellus (Fig. 2). Body measurements show little congruence among
them in their pattern of variation."
"Based on the
spatial congruence of the location of zones of morphological changes in the
three plumage characters evaluated, Scaled Woodcreepers may be divided into
three diagnosable population clusters Fig. 4): (a) one restricted to the left
bank of the São Francisco River, which from now on will be referred as wagleri
(Spix 1824); (b) one from the right bank of the São Francisco River to
around 22°S, which will be referred to squamatus (Lichtenstein 1822),
and (c) one from around 22°S to 30°S, which will be referred to falcinellus (Cabanis
& Heine 1859)."
"The changes
between the different types of each character are sharp and well defined. The
only exception is a single specimen (MPEG 45202, female) collected by one of us
(JMCS) in a locality on the left bank of the São Francisco River (Bahia, Coribe, 13°45'S, 44°28'W); the specimen has HEAD of type 2,
but BACK of type 1 (Fig. 3). "
"With the exception
of one single specimen (out of 11 specimens collected in the same locality)
that is within the range of wagleri but has HEAD similar to that of squamatus,
no other evidence of hybridization or intergradation among the three population
clusters was found. "
Silva &
Straube (1996) proposed considering wagleri, squamatus, and falcinellus
as three separate species applying the Phylogenetic Species Concept and the
Evolutionary Species Concept. Marantz et
al. (2003) provided further plumage features of all taxa and only partially
followed this proposition, splitting the three taxa in two species: wagleri +
squamatus on one side and falcinellus on the other.”
Genetic data
García-Moreno & Silva 1997 studied the phylogenetic
relationships of several Lepidocolaptes and wrote that "The average
uncorrected sequence divergence between the Lepidocolaptes species in
this study is 6.3% ± 2.6 (excluding L. lacrymiger, for which we lack the
full dataset, and the comparison between L. wagleri and L. squamatus,
which clearly belongs to a different time frame; see below and Table 2).
Assuming a substitution rate of 2% per million years (e.g., Klicka & Zink,
1997), they appear to be the product of a radiation that occurred between 1.9
and 4.5 million years ago (our estimates differ little whether we use data from
ND2, cyt b, or both fragments; see Tables 1 and 2). Using the same clock, but
only the cyt b data, we estimate L. lacrymiger to have split from L.
souleyetii and L. albolineatus about 2.3 mya (4.6% divergence),
which is well within the radiation of Lepidocolaptes. L. wagleri and L.
squamatus, with a difference of only 1.7% (although diagnosable by plumage
characters, see Silva & Sträube 1996), appear to
have split from each other more recently (850 000 years ago). " More
recently, Arbeláez-Cortés et al. (2012) used a concatenated dataset and found
that squamatus-wagleri (samples from Bahía) were sister to falcinellus (samples from Uruguay and Argentina), but distant
geographic samples, and partial genetic datasets precluded more detailed
analyses; no genetic distance was calculated for any gene between the three
taxa.
Intermediate specimens
(squamatus/wagleri and squamatus/falcinellus)
In
three other studies, Vasconcelos & D'Angelo Neto
(2009, 2018) and Vasconcelos et al. (2012), collected specimens in areas where squamatus
could meet with either falcinellus or wagleri. In the first study
(Vasconcelos & D'Angelo Neto 2009), at the Serra
do Juncal, on the border of Minas Gerais and São
Paulo, two specimens were collected. The authors expected these to be either falcinellus
or squamatus since this was an area where neither taxon had been
recorded. One specimen appeared to be typical of falcinellus while the
other an intermediate falcinellus-squamatus head pattern and ventral
pattern of squamatus. However, the
photographs of the specimens show more variation than expected, indicating that
this variation needs further assessment. In two other studies (Vasconcelos et al.
2012, Vasconcelos & D'Angelo Neto 2018) report
numerous intermediate specimens between wagleri
and squamatus from 8 wide ranging
localities (1 in Bahia and 7 in Minas Gerais, see Appendix 1 in Vasconcelos
& D'Angelo Neto 2018).
Figure 3 from Vasconcelos
& D'Angelo Neto (2009)

Figure 6 from Vasconcelos & D'Angelo Neto (2018)

Additionally,
Vasconcelos
& D'Angelo Neto (2009) elaborated on the
hybridization between squamatus and falcinellus:
"This is the first
record of intergradation between these two species (see Silva & Straube,
1996; Marantz et al., 2003). Furthermore, L. falcinellus was
known only from southern bank of Paraíba do Sul river (see Marantz et al., 2003)
and these records show that at least phenotypes of this species occurs north of
this river. The occurrence of phenotypes of L. falcinellus in the Araucaria
forests of the north slopes of Serra da Mantiqueira
can be related to palaeoecological connections to the
nucleus of Araucaria forests from southern Brazil (where L.
falcinellus is the only species of this complex – see Marantz et al., 2003).
In the Serra da Mantiqueira and adjacent regions,
expansion of Araucaria forests is hypothesized to have occurred between
9,700‐8,200 years before present and, later, after c. 3,500‐3,000 years before present, when
climate became cooler and moister than today (Behling, 1997, 1998, 2002; Garcia
et al., 2004). Curiously, L. squamatus is the taxa recorded at
Itatiaia, in the Atlantic (south) slope of this mountain range (Pinto, 1951,
1954). This could be explained by the occurrence of Araucaria forests in
the north slopes of Serra da Mantiqueira, whilst the
Atlantic slope is mostly covered by montane and cloud forests (see Hueck, 1972) and should has different zoogeographical
affinities."
A different
interpretation
We
interpret the available data in a different manner to Silva and Straube (1996)
and consider that the evidence indicates that there is a single, widespread,
biological species at stake. First of all, recently collected specimens have
shown that the presumed distributional limits of squamatus, falcinellus
and wagleri are different from those
proposed by Silva and Straube (1996), and that the presumed barriers do not
exist. Second, the fact that the plumage features exhibit stepwise leaps and
that squamatus appears to be
intermediate between wagleri and falcinellus in crown and back colour suggests that they are linked through a cline. It
might be a steep or a stepped cline, we do not know, but the very nature of the
typological analyses carried out by Silva & Straube (1996) does not seem
particularly suited to detect such a variation, while it will clearly be able
to detect the main geographical leaps in variation. Third, intermediate
individuals have been collected where each form meets the nearest taxon (with
numerous intermediates between squamatus
and wagleri, and few known
intermediates between squamatus and falcinellus), adding weight to the
argument that falcinellus and squamatus are conspecific.
Additionally, although no thorough
vocal analyses are available, the call (the most frequently heard and recorded
vocalization in this complex) is surprisingly similar across the geographic
range, with much variation within each taxon but nothing consistent that we
could detect among them (see the table above for some examples). We refrain
from analyzing songs here, as there appear to be few recordings and variation
is not well understood. Many recordings labelled as "song" in
Xeno-Canto and the MLNS pertain to calls, while few "songs" are
available for all taxa. Some voices seem different (perhaps not all homologues:
https://macaulaylibrary.org/asset/39093,
https://macaulaylibrary.org/asset/22502,
https://macaulaylibrary.org/asset/187233, https://www.xeno-canto.org/514658),
other seem identical (https://macaulaylibrary.org/asset/198642781,
https://www.xeno-canto.org/8183), and various other recordings at the Macaulay
Library seem misidentified (descending vocalizations; sound like Xiphorhynchus fuscus tenuirostris). With so few samples to compare and the
generally variable nature of long songs in Lepidocolaptes,
we cannot confidently assess what is going on. However, any such analysis would
need to include samples from across the presumed hybrid zones between taxa if
it is to rigorously test the species limits between them.
In sum, the lack of complete genetic
data, minor plumage differences linked through intermediates where their ranges
abut, and vocal similarities, we propose a return to the historical treatment
of falcinellus (and wagleri) as subspecies of squamatus.
We thank Vitor Piacentini for
questioning a previous, less detailed proposal, which prompted us to look for
further evidence on the taxonomic status of these woodcreepers, resulting in a
much more interesting and richer perspective.
Additional
references
Arbeláez‐Cortés, E., Navarro‐Sigüenza, A. G. &
García‐Moreno J. (2012). Phylogeny of woodcreepers of the
genus Lepidocolaptes (Aves, Furnariidae), a widespread Neotropical
taxon. Zoologica Scripta 41:
363–373.
García-Moreno, J. &
Silva, J. M. C. (1997). An interplay between forest and non-forest South
American avifaunas suggested by a phylogeny of Lepidocolaptes
woodcreepers (Dendrocolaptinae). Studies on Neotropical Fauna and Environment
32: 164–173.
Vasconcelos, M.F. &
D'Angelo Neto, S. (2009) First
assessment of the avifauna of Araucaria
forests and other habitats from extreme southern Minas Gerais, Serra da Mantiqueira, Brazil, with notes on biogeography and
conservation. Papeis Avulsos de Zoologia. 49: 49-71.
Vasconcelos, M.F., Souza,
L.N., Duca, C., Pacheco, J.F., Parrini, R., Serpa, G.A., Albano, C., Abreu, C.R.M., Santos, S.S. &
Fonseca-Neto, F.P. 2012. The avifauna of Brejinho das Ametistas, Bahia,
Brazil: birds in a caatinga-cerrado transitional zone, with comments on
taxonomy and biogeography. Revista
Brasileira de Ornitologia 20: 246-267.
Vasconcelos, M.F. &
D'Angelo Neto, S. (2018) First avifaunal survey of a Cerrado dry forest enclave on the right bank
of the São Francisco River, Minas Gerais, Brazil, with insights on geographic
variation of some species. Papeis Avulsos de Zoologia
58: e20185815
Juan I.
Areta and Mark Pearman, August 2020
Comments from Stiles: “YES to placing falcinellus as a subspecies of squamatus.
Clearly, more data on distribution and a genetic analysis of haplotype
sharing are needed, and retaining all three subspecies as part of a single
species seems the best course with the data available.”
Comments
from Pérez-Éman:
“This proposal aims to treat Lepidocolaptes falcinellus as a
subspecies of L. squamatus. The basis
for this lump is summarized at the end of the proposal and it includes four
main points: the lack of complete genetic data, minor plumage differences,
intermediate forms in the contact zones, and vocal similarities. I think
decisions could go either way depending on the importance given to particular
criteria and/or the presence/lack of information. My take on each of the four
main points follows.
“1. Molecular sequence data
available for these taxa is few and widely scattered throughout the literature.
Arbeláez-Cortés et al. (2012) used concatenated sequences of both mitochondrial
cytochrome oxidase subunit 1 (CO1) and NADH subunit 2 (ND2) genes (with
sequences of each gene coming from different individuals, known as chimeric
sequences) to estimate the phylogenetic relationships among species in the
genus Lepidocolaptes. For falcinellus, sequences came from
individuals of both Misiones (Argentina) and Cerro Largo (Uruguay), whereas,
for squamatus, both of them came from
Bahia, but from either side of the San Francisco River (Coribe
and Palmas Monte, Bahia, Brazil), potentially including individuals of both wagleri and nominal squamatus. This strategy might have not influenced the phylogenetic
goals of the study but certainly might mask some of the genetic variation
between these two taxa. In fact, preliminary and rather short sequences
(cytochrome b and ND2 genes) from the same squamatus
individuals were produced by García-Moreno & Silva (1997) but were
identified as both wagleri and squamatus (and showed a moderate genetic
divergence; see below). It is worth to notice that the only individual with
intermediate plumage characters between these two taxa, reported by Silva &
Straube (1996), was found in Coribe, Bahia (Brazil).
Other CO1 gene sequences available are from Chaves et al. (2015, Molecular
Ecology Resources) and Klippel et al. (2015, PLoS ONE), both coming from work
on DNA barcoding. Chaves et al. (2015) sequence is from an individual collected
in Brejinho das Ametistas, Caetité (Bahia, Brazil) and the Klippel et al. (2015) is
from northern Espiritu Santo. Unfortunately, it seems to be unclear how to
label both Coribe and Caetité
individuals, as intermediate individuals have been found in both localities at
either side of the San Francisco River in Bahia (Silva & Straube 1996,
Vasconcelos & D´Angelo Neto 2018). Information on
phylogenetic relationships and genetic divergence can be extracted from these
sequences. Arbeláez-Cortés et al. (2012) phylogenetic hypothesis of the genus Lepidocolaptes, based on the
concatenated dataset (CO1+ND2), showed a strongly supported sister-taxon
relationship between squamatus and falcinellus, a result that is congruent
with results based on just ND2 sequences or genomic data (UCEs) (posterior
probabilities of 1.0 in Bayesian analysis and Maximum Likelihood bootstrap
support of 100; see my comments on Proposal 868 that include both phylogenetic
hypothesis). An uncorrected genetic divergence of 1.7% between wagleri and squamatus, based on both cytochrome b and ND2 genes, was reported
by García-Moreno & Silva (1997); however, these data came from very short
sequences a bit longer than 200 base pairs (a normal sequence length obtained
at that time). Unfortunately, these are the only two samples sequenced for
these genes. For CO1, uncorrected genetic divergence ranges between 0.5 and
1.5%, with the northern Espiritu Santo sample the most divergent, but keep in
mind the Bahia samples came from a contact zone with reports of intermediate
plumage individuals. ND2 uncorrected genetic divergence between falcinellus and squamatus averaged 4.35% (only two individuals for each taxon, with
the additional falcinellus coming
potentially from Brazil (Rodrigues et al. (2013) but collection locality
requires the missing Supplementary Information on this article). Divergence
between both falcinellus samples was
0.4%. How does this genetic divergence between falcinellus and squamatus
compare to other species pairs? These are five examples of average uncorrected
genetic divergence: affinis/leucogaster = 4.2%, angustirostris/souleyetti = 4.6%, albolineatus/angustirostris = 4.5%, fatimalimae/angustirostris = 4.7%, and angustirostris/squamatus = 4.0%. Average uncorrected
genetic distance within the genus is approximately 7%, a bit higher than the
6.3% previously reported by García-Moreno & Silva (1997) given that L. lacrymiger, the most divergent
species (approx. 7%) was not included in such calculations. In summary,
molecular data support a sister-taxon relationship between falcinellus and squamatus
(including here both wagleri and
nominal squamatus), and uncorrected
genetic divergence between both taxa is at the level of several species pairs
within Lepidocolaptes; a lower
divergence is found between potential individuals of both wagleri and squamatus.
“2. Plumage differences among
the three taxa (wagleri, squamatus, and falcinellus) seem to be minor. However, based on Silva &
Straube (1996), they are consistent at the broad spatial scale they were
evaluated. L. falcinellus differed
from the first two taxa in the complete set of three characters, while wagleri and squamatus just in head and back plumage pattern and coloration
(similar in tail coloration). However, more recently, Vasconcelos &
D´Angelo Neto (2009, 2018) found individuals with
intermediate plumage patterns in two of the potential contact zones. Fourteen
(14) out of 29 individuals of squamatus
(and wagleri) collected mostly at the
right bank of the San Francisco River showed intermediate plumage patterns (at
both sides of the river). A similar pattern, though with less number of
individuals, was found in Serra da Mantiqueira where
individuals of the falcinellus
phenotype were collected, known previously only to the south of the Paraíba do
Sul river. Reading the description of these intermediate forms, you can come to
the conclusion that plumage comparative studies need to include more characters
than the originally considered by Silva & Straube (1996); for example,
underpart colors (breast and belly), have shown to be very informative when comparing
variation among these taxa and were not considered by these authors. Besides,
such descriptions should be thorough and emphasize contact zones and the
geographical distribution of such intermediate phenotypes.
“3. I have already commented
on the intermediate forms issue. These findings suggest hybridization between
these taxa (currently or in the past) and, if so, clearly shows that the
potential riverine barriers associated to geographical limits of these taxa are
not unsurmountable or physical/climatic conditions have been dynamics through
the history of the region. Environmental conditions have changed and have
affected many taxa in the Atlantic Forest Region, a pattern that is clearly
shown in the literature. Studies cited by Vasconcelos & D´Angelo Neto (2009) indicate that, at least, the area with
potential gene flow between falcinellus
and squamatus has been clearly
dynamic in the recent history. This information, however, does not help to
understand the dynamics of this contact/hybrid zone. Is there current gene
flow? Is symmetric or asymmetric? is the hybrid zone expanding or forces such
as natural selection are counteracting a potential expansion, such as the
findings recently found for the Baltimore and Bullock´s oriole hybrid zone by
Walsh et al. (2020, Auk)? I think we have an interesting setting to expand on
these findings and conduct a morphological/molecular/behavioral study that
provide with some answers to these questions.
“4. Vocal
similarities/differences are the weakest point here because there is no much
information and the available one is complex to evaluate because of its
variable nature. In fact, these were the reasons the proposal did not provide
any vocal analyses. However, Vitor Piacentini´s
comments on the differences among songs of these taxa and the distinct use of
their repertoire, even when not evaluated quantitatively, clearly suggest that
there is a story beyond what is currently available that needs to be evaluated
before taking any taxonomic decision.”
“In summary, falcinellus and squamatus are sister taxa with a level of divergence comparable to
many species pairs within the genus Lepidocolaptes.
Even when genetic distance does not represent a yardstick to diagnose
speciation events, such a level of divergence suggest a long evolutionary
history in isolation. At this point, one still could go either way and look for
information in other characters. Plumage pattern though minor are consistent
(apparently) in large ranges of their distribution and intermediate plumages
are concentrated (so far) in contact zones (more extensive for wagleri/squamatus with intermediate forms found at both sides of the San
Francisco River in Bahia and Minas Gerais, Brazil). If these intermediate forms
are evidence of hybridization, the only existence of an hybrid zone does not
invalidate the recognition of species level for both taxa. We need to study the
dynamics of such hybrid zone. A pattern that requires further study is the
potential altitudinally replacement pattern, when in parapatry in Rio de
Janeiro, with falcinellus occupying
the upper portion of the gradient (Piacentini´s
comments). Finally, a vocal study is missing and the lack of evidence is not
evidence. The pattern found for these two taxa is very similar to the one found
for Myrmoderus squamosus/loricatus (study case mentioned by Vitor
Piacentini): sister taxa, a potential origin associated to vicariant geological
events previous to the Pleistocene and a dynamic demographic history due to a
recent history of climate changes (Raposo do Amaral et al. 2013, Molecular
Ecology). These Myrmoderus species
are somehow similar in plumage pattern and vocalizations with a replacement
zone associated to the Paraíba do Sul river. Some (few) hybrids have been found
but apparently minor differences in songs have a strong effect in species
discrimination, competition, and possibly mate recognition (Macedo et al. 2019,
Behavioral Ecology). In conclusion, my position is similar to the one expressed
by Vitor Piacentini and, for the time being, is to keep falcinellus and squamatus as
different species with this last taxon including both nominal squamatus and wagleri (the Silva & Straube (1996) proposal considering the
Biological Species Concept). There is no clear available evidence so far that
justify a treatment in which these two taxa are better considered as a single
species. There are important gaps that need to be filled if we want to make
informed decisions. It is true that we also lack the evidence to treat them,
confidently, as different species but a return to a historical one species treatment
without evidence is to risk the need for further adjustments in the near
future.”
Comments from Claramunt: “NO. I think that the
interpretation in the proposal is reasonable, but we need to see some actual
evidence. Is variation clinal? We need to see plots and maps. Is there a hybrid
zone between squamatus and falcinellus? It seems so, but is the hybrid zone wide and
with lots of intergrades or narrow and composed of sterile F1s? Narrow hybrid zones are not evidence of
conspecificity in modern species concepts. I think that a published paper is
needed to flesh out the required information.”
Comments
from Robbins:
“I’m on the fence on this one. If we
hadn’t already recognized falcinellus as a species I would lean towards
treating it as a subspecies of squamatus given the incompleteness of
some of the data sets. However, instead
of flipping back and forth, like Jorge and Vitor, I’m for continuing our
current treatment until there are additional data to suggest otherwise.”
Comments from Zimmer: “NO. I’m in complete agreement with Vítor’s comments on this topic. His observations regarding differences in the
songs (as opposed to calls) of falcinellus
relative to those of squamatus, and,
especially, to the behavioral differences between the two in the relative
frequency with which the two taxa use songs versus calls, squares very well
with my experience. I have little doubt
that a quantitative vocal analysis of songs of falcinellus and squamatus
would reveal diagnostic differences in more than one vocal character, but Areta
& Pearman admit in their Proposal, that they excluded songs altogether from
their analysis due to small sample sizes of recordings of songs, and variation
in those small samples that was “not well understood.” Even the comparison of calls across taxa
cited in the Proposal appears to have been a qualitative comparison rather than
a quantified, thorough analysis. I agree
that the calls of the three taxa (including wagleri)
in this complex are quite similar to the human ear (as are the calls of some
other Lepidocolaptes, such as angustirostris), but I would not be at
all surprised to find that spectrographic analysis might reveal diagnostic
between-taxon differences in the note shapes, degree of frequency modulation,
peak frequencies and note width (duration) of those calls, even if those
differences are not readily apparent to the human ear. Differential usage frequency within vocal
repertoires is also important, as Vítor suggests. [As an aside, I would liken the difference in
usage frequency of songs versus calls between falcinellus versus squamatus
to that seen between N bank (of Amazon) versus S bank populations of Lophotriccus galeatus. N bank galeatus
seem to deliver lengthy or intermittent series of “tic” notes as their primary
vocalization, only occasionally reverting to the trilled, gravelly song. S bank populations on the other hand,
routinely deliver the trilled song, with only occasional “tic” calls as
punctuation. Similar geographic
differences in usage rates of analogous/homologous vocalizations have also been
noted in populations of the Zimmerius gracilipes/acer complex, the
members of which are now treated as distinct species.] The point is, no thorough analysis of
vocalizations in this complex has been undertaken, and given consistent plumage
differences and the genetic distance between falcinellus and squamatus
(which, as Jorge notes, reveals comparable levels of divergence to that seen in
other species-pairs in Lepidocolaptes,
and suggests a long evolutionary history in isolation), I would argue that the
burden of proof for producing such an analysis would fall on those arguing for
a one-species treatment, particularly in light of the anecdotal observations
presented by Vítor and myself regarding differences in songs (available for
qualitative comparison in publicly accessible sound archives, such as
Xeno-canto), and differential song versus call frequency within
repertoires. The number of Lepidocolaptes specimens from the
contact zone in Bahia and Minas Gerais exhibiting plumage characters seemingly
intermediate between wagleri and squamatus, adds further weight to the
treatment advocated by Marantz et al. (2003), who considered wagleri and squamatus as conspecific (while treating falcinellus as a separate species).
The scarcity of such intermediates between falcinellus and squamatus
from the contact zone, along with the elevational parapatry of the two taxa in
southern Rio de Janeiro, bolsters the case for continuing to recognize two
species, and suggests that any “hybrid zone” is very narrow. To me, falcinellus
is very much a bird of the upland Araucaria-Podocarpus forests that typify
higher elevations of Paraná, Santa Catarina and Rio Grande do Sul, and, as
such, is an ecologically different beast from squamatus/wagleri, an impression that is only reinforced by the
elevational parapatry of the two species in the Serra da Bocaina
as described by Vítor.”
Comments
from Jaramillo:
“NO – I will admit I am most influenced by Kevin’s analysis of the situation
here, thanks Kevin for your thoughts. On the other hand, the proposal is a
reasonable proposal. There is clearly a need for more data, in particular song
as noted. I am usually not the person who asks for more data, I tend to recoil
at the thought because often this is kicking down the road a problem that we
already can act on to a later date due to a bit missing details on only part of
the problem. In this case, I think the available data can be interpreted in two
ways, essentially it is on the fence, rather than there being an obvious
choice.”
Comments
from Lane:
“NO. I am swayed by Kevin's and Vitor's
comments, and believe the prudent stance is to maintain the current status quo
until additional fieldwork addressing the remaining issues is conducted and
results presented.”
Comments from Pacheco: “NO. In this case, I have a
position similar to Kevin and Dan. It is preferable to wait for additional
field data to resolve the treatment given here.”
Comments from Bonaccorso: “NO. More evidence is needed to
decide, so we don´t have to revert this decision in a few years. A 4%
difference in mitochondrial DNA suggests that there might be some genetic
differentiation in fast introns or SNPs; looking into this type of evidence may
help solve the issue. Also, more quantitative data on vocalizations is needed.
I am not worried about intermediates if they are confined to a narrow hybrid
zone. I think this is the most conservative approach to the issue, at least
until more evidence is available.”