Proposal (907) to South
American Classification Committee
Split Red-eyed Vireo (Vireo olivaceus) into two species
Note from Remsen: Below is a proposal that was passed by NACC and is posted here with
permission from its authors. It was
included in the 59th Supplement (Chesser If it passes, it would
result in SACC treating boreal migrant populations as one species: Red-eyed
Vireo (V. olivaceus) and Chivi Vireo (V. chivi).
Background: Current taxonomy recognizes the Red-eyed Vireo
(Vireo olivaceus) as one species with
two allopatric groups during the breeding season, which become sympatric during
the nonbreeding season (AOU 1998). Ridgway (1904) referred to the species as
monospecific, but a long history of debate has surrounded this species and the
complex of related species. The two allopatric groups are known as olivaceus and chivi. The olivaceus group
includes one or two migratory subspecies that breed in North America and spend
the winter in South America. The chivi group
includes nine subspecies from South America that consist of sedentary and
migratory populations (Cimprich et al. 2000). The
main reasons why both groups have been referred to as subspecies are their
subtle plumage differences and the eye color of adults, which is red in olivaceus and brown in chivi (Johnson and Zink 1985).
Johnson and Zink (1985), using starch gel
electrophoresis, showed that the two geographically disjunct groups of the
Red-eyed Vireo are conspecific. In that study they included 17 olivaceus samples from North America, 14
chivi samples from Paraguay (only the
diversus subspecies), 1 sample of V. flavoviridis, and 1 sample of Cyclarhis gujanesis as an outgroup.
Subsequently, Slager et al. (2014) reconstructed a phylogeny of the Vireonidae
family using the complete mitochondrial gene ND2, which suggests that the two
disjunct groups of V. olivaceus do
not represent sister clades. In their phylogeny, the North American lineage is
more closely related to populations of V.
flavoviridis from Yucatán, Mexico, whereas the South American lineage is
more closely related to V. altiloquus.
Slager et al. (2014) concluded that the reciprocal monophyly recovered by
Johnson and Zink (1985) might represent an artifact of incomplete taxon
sampling. However, they recommended analyses using more loci to fully resolve
the species relationships.
New Information:
Battey and Klicka (2017) published a
phylogenetic study of the Red-eyed Vireo species complex. The aim of this study
was to identify cryptic species and to assess rates of gene flow in a lineage
that includes migratory species that alternate between sympatry and allopatry
during an annual cycle. Battey and Klicka (2017) analyzed 40 individuals and 6
species of Vireo, which included four
members of the Red-eyed Vireo complex: V.
olivaceus, V. flavoviridis, V. altiloquus, and V. magister; and two outgroup taxa: V. gilvus, and V. plumbeus
(Figure 1). They obtained genetic data following the ddRADseq
protocol, which resulted in a final dataset of 38 individuals with an average
of 13,323 loci per individual. They inferred a maximum likelihood phylogenetic
tree(RAxML v8) and a species tree (SNAPP v. 1.3). They also conducted
clustering analysis (STRUCTURE), Principal Components Analysis (Adegenet), and admixture analysis using D statistics.
Phylogenetic analyses revealed that northern
and southern olivaceus are
paraphyletic, with South American breeders more closely related to the
Caribbean taxa altiloquus and magister than to their North American
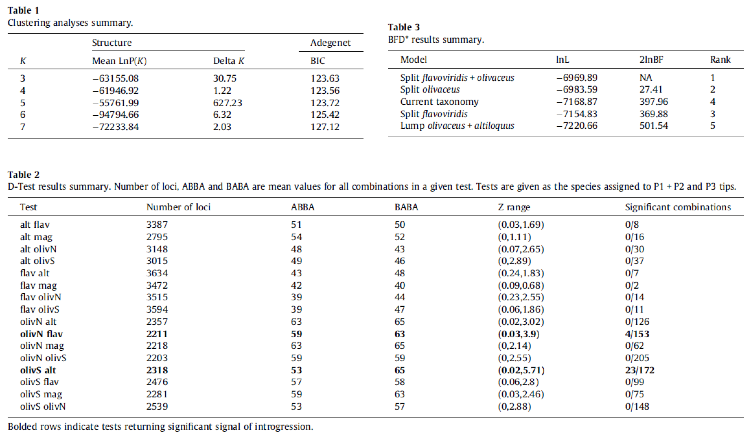
conspecifics. The STRUCTURE analysis favored a five-population model (Table 1)
that split northern and southern olivaceus.
It should be noted that both clustering analyses, STRUCTURE and Adegenet, showed a tendency to lump northern and southern olivaceus when run at k = 4.
D statistics did not support significant
introgression between northern and southern olivaceus
populations. The Bayes factor delimitation analysis favored the models that
split northern and southern olivaceus
(Tables 2 and 3).
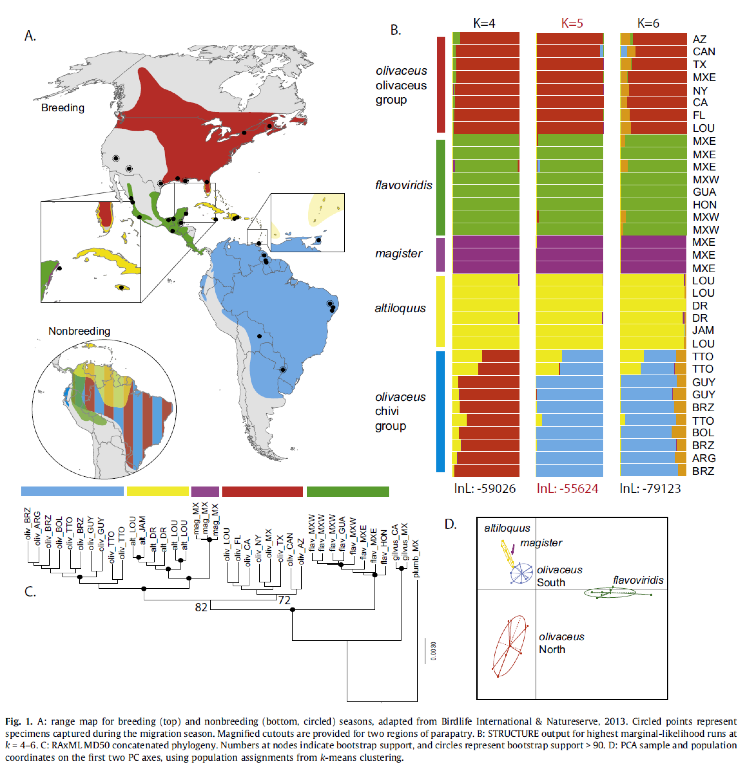
Battey and Klicka (2017) concluded that olivaceus includes two genetically
divergent lineages breeding in disjunct ranges. Life history, in addition to
genetics, also supports splitting the species. Northern (olivaceus) and southern (chivi)
populations are non-monophyletic, do not exchange genes, and have different
direction and timing of migration, which are heritable life-history traits and
confer reproductive isolation between the groups. The authors propose elevating
the chivi group (all populations
breeding in South America) to species status under the English name Chivi
Vireo, based on the scientific name.
Recommendation:
We recommend splitting Vireo olivaceus into two species.
North
American populations: Vireo olivaceus,
Red-eyed Vireo
South
American populations: Vireo chivi,
Chivi Vireo
Literature Cited:
American Ornithologists' Union.
1998. Check-list of North American birds. 7th edition. Washington, D.C.:
American Ornithologists' Union.
Battey, C. J. and J. Klicka. 2017.
Cryptic speciation and gene flow in a migratory songbird species complex:
insights from the Red-Eyed Vireo (Vireo olivaceus). Molecular
Phylogenetics and Evolution113:67-75.
Cimprich, D. A., F. R. Moore, and M. P. Guilfoyle. 2000. Red-eyed Vireo (Vireo olivaceus). The Birds of North America (P. G. Rodewald, Ed.). Cornell
Lab of Ornithology, Ithaca, NY. https://birdsna-org.libproxy.berkeley.edu/Species-Account/bna/species/reevir/
Johnson, N. K. and R. M. Zink.
1985. Relationships among Red-eyed, Yellow-green, and Chivi vireos. Wilson
Bulletin 97:421-435.
Ridgway, R. 1904. The birds of
North and Middle America. Bulletin of the U.S. National Museum, no. 50, part 3.
Slager, D. L., C.J. Battey, R. W.
Bryson Jr., G. Voelker, and J. Klicka, 2014. A multilocus phylogeny of a major
new world avian radiation: the Vireonidae. Molecular Phylogenetics and
Evolution 80:95-104.
Submitted by: Rosa Alicia Jiménez and Carla Cicero, Museum
of Vertebrate Zoology (to SACC February 2021)
Comments from Remsen [this is what I wrote on the NACC
proposal]:
“YES. The conclusions assume that
all South American vireos are part of the V. chivi complex. As noted in the paper, the V. chivi
group consists of highly migratory and totally sedentary subspecies, the latter
largely restricted to riverine habitats (and thus the range map in the paper
vastly over-states the actual range and especially the continuity of the
breeding distribution). Yet the paper
treats this complex as a monolithic unit based on what looks like only 9
geographic samples from what appears to be no more than 4 subspecies (for some
reason I cannot access Table 1 in Supplemental Material), none of them from
northwestern South America, where 4 unsampled subspecies also include isolated
trans-Andean caucae and griseobarbatus. Thus, the taxon-sampling failed to include
the majority of diversity in the complex (although Dave Slager’s
paper covers this better). The
assumption that these are all to closer nominate chivi than anything
else is probably safe but rests on traditional but untested boundaries in the
complex. So, I worry. The conclusion (that olivaceus is
paraphyletic with respect altiloquus depends entirely on one node in
Fig. 1C, and yet the support for monophyly of the morphologically uniform olivaceus
group is weak. Finally, altiloquus
itself consists of 6 subspecies, of which no more than 2 were sampled.
So,
I asked Mike Harvey for an assessment of that node, and his response (quoted
here with permission) soothes my reservations:
“First off, I would perhaps put less stock in the concatenated tree
than the STRUCTURE results and perhaps the SNAPP tree. As you know, trees from
concatenated genes can produce wonky results when gene trees are heterogeneous.
We have reason to expect the gene trees in this case are heterogeneous, both
because the mtDNA tree differs dramatically from the nuclear trees (suggesting
at least some past horizontal gene flow) and because the nodes in the
concatenated tree that are poorly supported are near each other deep in the
tree, suggestive of mixed signals due to competing topologies in that part of
the tree. However, although the likely explanation for those low support values
is ancient hybridization, this occurred well in the past and I don't think it
in any way indicates that northern and southern olivaceus are not
distinct. The STRUCTURE results suggest no recent admixture between the two
populations, and the SNAPP tree suggests they aren't sister at most of the
genome. Given strong support for the monophyly of (S olivaceus+altiloquus+magister)
in both concatenated and SNAPP trees, I doubt there has been any admixture
between the two olivaceus populations since olivaceus split from altiloquus and
magister, thus they are unlikely to be sister at any part of the genome.
The only small caveat here is that the sample sizes of individuals aren't huge,
although they aren't horrible either. I doubt the inferences about olivaceus
would change even with more individuals.”
Also, note that treating V.
gracilirostris as a species likely makes broadly defined V. chivi a
paraphyletic taxon
Comments
from Lane:
“A reluctant YES. The Harvey assessment of the trees
seems to support the age of the split of North American Vireo olivaceus and
South American V. chivi groups, but as noted by Van, it is
incredibly frustrating that Battey and Klicka (2017) ignored samples from along
the Amazon and NW South America (despite there being plenty of samples
available!). These populations would be very informative to include if only to
see what structure they would add to the chivi clade. The Slager et al
(2014) tree uses more samples, but has some strange and conflicting tree
topologies with respect to the monophyly of V. flavoviridis and
all remaining forms of the "V. olivaceus" clade!
“The morphology and voices of the populations of Vireo chivi
I know suggest that there is far more to the story than just splitting V.
olivaceus into two species and being done with it. Indeed, populations
currently considered nominate "chivi" are quite variable
themselves (leading me to wonder how many unrecognized taxa there are?). The
type locality of chivi is western Paraguay (not SE Brazil, as I would
have guessed, which is actually subspecies diversus), and thus the name
is probably best applied to the migratory population of the drier interior
Chaco and Chiquitano woodlands and nearby Andean foothills of Paraguay,
Bolivia, and Argentina. There are populations farther west in intermontane
valleys that are variably migratory (particularly in the more deciduous
valleys) and resident (in more humid valleys such as the Urubamba at Machu
Picchu) that are generally considered "chivi" for lack of any
additional names. Interestingly, to my ears, songs differ strongly between
birds from Santa Cruz, Bolivia, and birds east into Brazil (Minas Gerais and
Sao Paulo, which share a rapid quavering character in song elements with Santa
Cruz), and those that breed in deciduous forests of La Paz, around Machu
Picchu, and the deciduous valley of the Mantaro farther north in Peru. The
resident solimoensis of the main Amazonian
tributaries is comparatively distinctive again, giving a particularly slow
paced song with little variation in song elements. Interestingly, I think most
South American forms differ from Nearctic-breeding olivaceus in having
less diversity and complexity in song elements and generally slower delivery of
notes, although this appears not to be the case for the two forms from NW Peru
and S Ecuador (which sound much more like V. flavoviridis fide
Moore et al.'s Ecuador bird song DVD). I'd be interested to know if others
familiar with singing South American birds have noticed the same?”
Comments
from Areta:
“YES. I agree with concerns expressed by Van and Dan. Although there is more
complexity in South America than what available studies show, it seems
untenable to keep olivaceus and chivi as part of the same species.
Whether the remainder of South American "Chivi" vireos really belong
to the Chivi-group and whether more species can be recognized remain as open
tasks. The situation in Ecuador and Peru (with cis and trans Andean
populations, lowland resident, northern and southern migrants, including taxa griseobarbatus, solimoensis, pectoralis, olivaceus and
chivi) seems particularly complex and
begs for more rigorous studies of seasonality, vocalizations and genetics (see
for example Ridgely & Greenfield 2001 Volume 1, and Schulenberg et al.
2007).”
Comments
from Bonaccorso:
“YES. It makes sense a lot of sense from the
genetic data; these clades are not even monophyletic! These data, together with
the information about the natural history of these broad groups, complete the
picture of two different lineages. I agree that there is a lot of additional
complexity in South America, but I doubt it will mean reverting this change. We
just need a better understanding of diversity within Vireo chivi.”
Comments
from Stiles:
“YES, the split V. chivi from V. olivaceus, clearly justified by
the genetic data. That the data presented might miss differentiation in the chivi
group is another story, but genetic samples (or recently-taken specimens
for toepads from several countries, including Colombia, are available, so
enough material is available for the asking to do a decent job of writing the
next chapter!”
Comments
from Claramunt:
“YES. The genetic evidence is convincing, in
particular the fact that olivaceus and chivi are not even sister
species.”
Comments
from Robbins:
“YES,
based on the Battey and Klicka genetic data, this is a straightforward decision
for recognizing olivaceus as a separate species from chivi.
However, as all of us know who have worked across the SA continent, there are
very likely more cryptic species to recognize within chivi. This is a
first step.”
Comments
from Pacheco:
“YES. Although the sample coverage of the work is not
as wide as it could be, I am of the opinion that the molecular data are consistent
with the treatment of two species/two lineages.”
Comments
from Jaramillo:
“YES, it is a start. I would think the migrant vs
resident populations are going to show some interesting dynamics. They look
visually quite different as well, although I have only seen a few of the
residents.”