Proposal (921) to South
American Classification Committee
Treat Trogon rufus
(Black-throated Trogon) as consisting of five species, including one newly
described
Effect
on SACC list: This proposal would treat our Trogon rufus
as consisting of five species, one of which is newly described.
Background: Our current Note reads as follows:
7c. Dickens et al. (2021) found evidence
that T. rufus should be treated as five separate species, including one
newly described: Trogon muriciensis of the Atlantic Forest patches of
northeastern Brazil; they recommended elevating the subspecies tenellus,
cupreicauda, and chrysochloros to species rank. SACC proposal badly
needed.
Trogon rufus (Black-throated
Trogon) is a polytypic species with one of the largest distributions of any
trogon, with taxa treated as (nine described) subspecies occurring in three disjunct
regions: (1) from Honduras to the Chocó of northwestern South America, (2)
Amazonia; and (3) the Atlantic Forest from Alagoas through Brazil to eastern
Paraguay and Misiones, Argentina. They
have all been treated as conspecific from Cory (1919; albeit as T. curucui
due to early name confusion), Pinto (1937), and Peters (1945) through the
present.
Here's
the map (from Dickens et al. 2021), which will be useful in evaluating the
proposal:
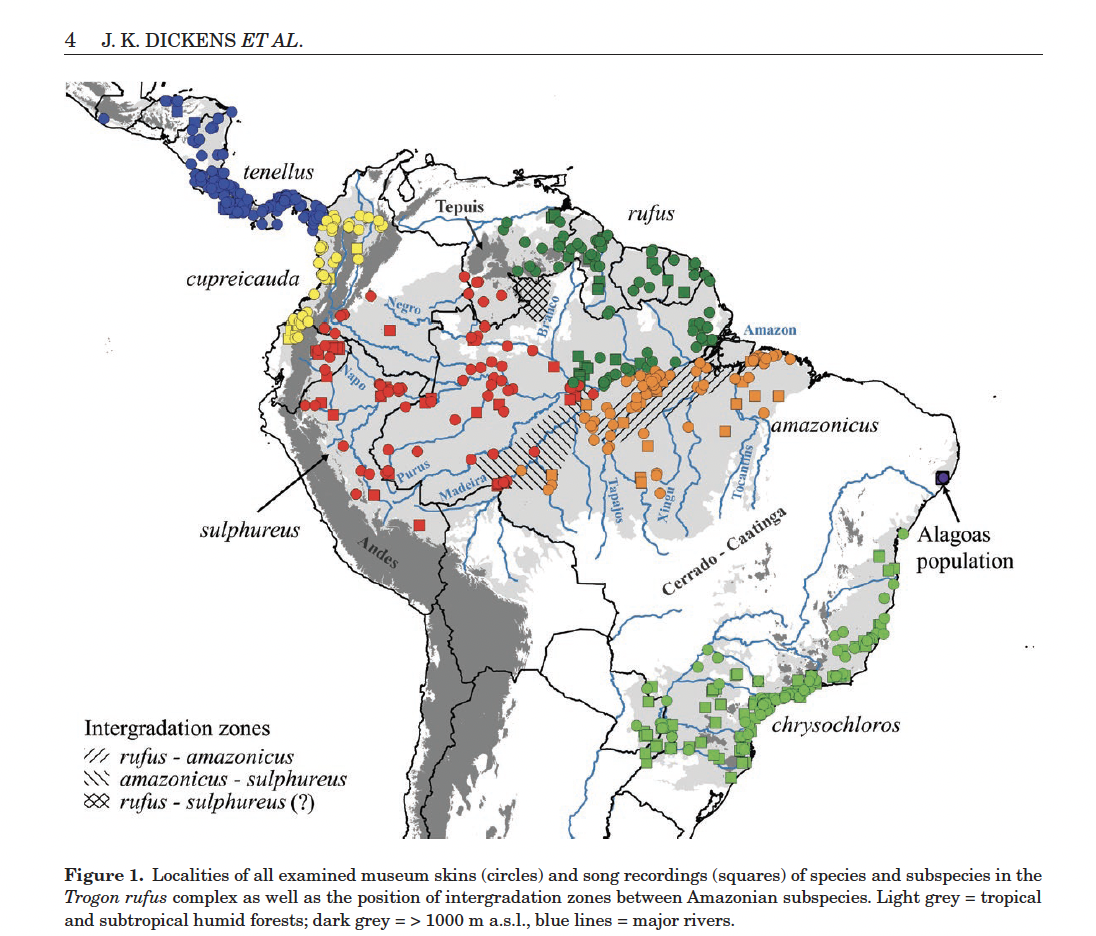
New
information:
Jeremy Kenneth Dickens and colleagues examined 906 specimens at 17 different
museums, including all taxa and all available type specimens, and gathered
spectrophotometric data, patterning, bare parts coloration, and standard
morphometric data on subsets of these specimens. They quantified vocal characters from 273
songs from throughout the distribution and including all named taxa. They also analyzed genetic samples (ND2 and
Cyt-b) from 29 specimens from throughout the distribution and all taxa. In other words, Dickens, Britton, Bravo, and
Silveira conducted an amazing study in terms of sample size, geographic
coverage, and critical data. Although
the genetic sampling included only mtDNA, I don’t think data from additional
genes would have made a difference in terms of determining species limits. The analyses are really excellent, and I
encourage everyone to check out the great graphics (my favorite is Fig. 3a on
male uppertail covert hue, although the color illustrations by Eduardo Brettas of
the taxa and their critical features is tough to beat). If only we had papers of this quality and
depth, from sophisticated analyses to classical taxonomy. The only weakness, given the vocal
differences, is the absence of playback trials, although to do that properly
would require fieldwork in multiple regions in the Neotropics.
A
detailed synopsis of all these data would take up a lot of space here. Check out the details for yourselves, but
here’s what stands out to me: the voices of all their proposed species are
qualitatively and quantitatively different; in contrast, no such major
differences are found among the Amazonian taxa that they recommend be treated
as subspecies of T. rufus sensu stricto.
For three of their proposed species-level taxa, 100% of the recordings
were correctly classified to species using linear discriminant analysis for
three of their proposed species-level taxa (chrysochloros, cupreicauda,
tenellus).
As
for the genetic data (ND2 1041 bp, cytb1011 bp), the results show prefect
congruence between geographic samples and relationships, and genetic distances
within geographic clusters and within taxa are small. The cis-Andean taxa are all weakly
differentiated, with time calibrations suggesting divergence times among
species at ca. 3-4 million years. The
big break is between cis-Andean and trans-Andean taxa, with a divergence time
estimated at ca. 5 million years ago.
The single sample of cupreicauda is fairly divergent from the 9
samples of tenellus.
(There
are lots of little nuggets within this 42 page paper that I could itemize, but
I will stop at just one because it has direct relevance to species limits. Atlantic forest chrysochloros is
almost exclusively insectivorous, in contrast to the other omnivorous taxa, and
it has a more heavily serrated bill for grasping large arthropods; they are
also known to be regular followers of monkeys, army ants, and coatis, evidently
more so than other trogons, so this all fits.
Actually, I can’t resist adding a second one because as the authors
note, it is important to keep in mind when assessing plumage in trogons: they
found evidence that there may be an environmental influence on iridescence,
which changes with elevation in chrysochloros.)
A
summary of their recommended species classification is as follows. See the paper for detailed diagnosis of each,
including coloration, pattern, eyering color, and song features. These sections also contain detailed
descriptions of each taxon, detailed synonymies, and useful notes on type
specimens
• Trogon tenellus Cabanis, 1862: Central
America to extreme NW Colombia (dpto. Chocó)
• Trogon cupreicauda (Chapman, 1914: N
Colombia south on Pacific slope to NW Ecuador
• Trogon rufus Gmelin, 1788 (including
nominate rufus of Guianan Shield, T. r. sulphureus of western
Amazonia, and T. r. amazonicus of eastern Amazonia)
•
Trogon muriciensis sp.
nov.: Alagoas Forest region; known only from type locality at Estação Ecologica de Murici.
• Trogon chrysochloros Pelzeln, 1856: Atlantic
Forest region
Here is a screen shot of the outstanding color
plate by Eduardo Brettas that illustrates the taxa:
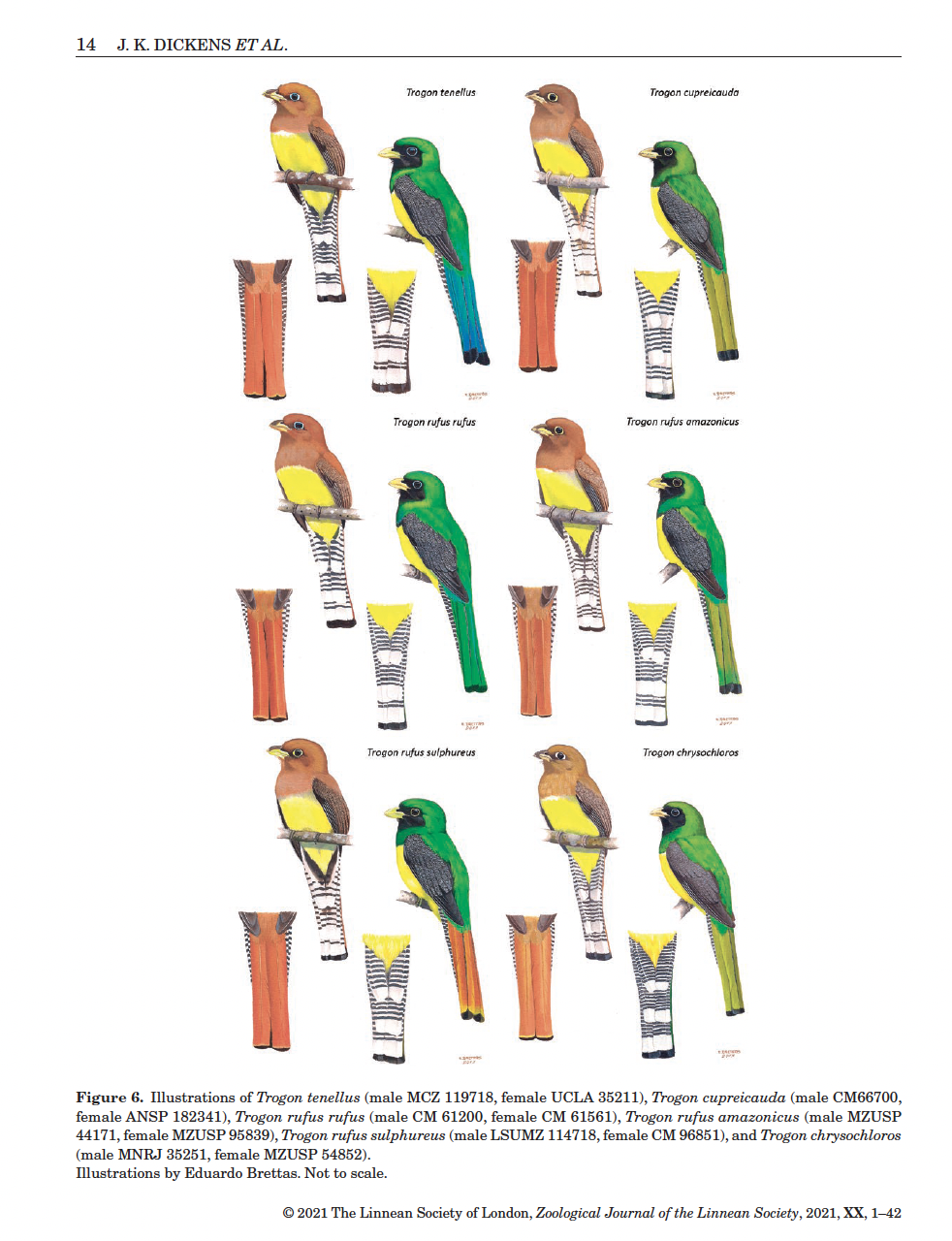
The quality of evidence for species rank varies
among the 4 newly recognized or new species, so I think we should subdivide the
proposal as follows below. I suggest
listening to the recordings of the various taxa on xeno-canto: https://www.xeno-canto.org/explore?query=trogon+rufus&pg=1
A.
Treat tenellus as a separate species from T. rufus
Because
tenellus is entirely trans-Andean, it is not parapatric with any
subspecies of T. rufus, and thus the decision on taxon rank must rely on
comparative methods. The vocal
differences from other taxa are given as follows:
“Song: Diagnosed from neighbouring T.
cupreicauda by fewer notes per phrase, longer note durations and generally
higher note frequencies, particularly the introductory note high frequency.
Note frequencies, particularly the introductory note high frequency, are higher
than for T. rufus subspecies. Fewer notes per phrase, slower pace and
longer durations of notes and pause following introductory note than T.
chrysochloros.”
In
terms of phenotype, It has a breast band, unlike T. r. sulphureus or T.
r. amazonicus, from which it differs in several other less conspicuous
details (p. 26, and see the color plate (Fig. 6, p. 14, and above). The Diagnosis states that its pale (blue-gray
to white) eyering that distinguishes is from everything else, including
parapatric cupreicauda, but chrysochloros also is listed as
having the same color eyering.
B.
Treat cupreicauda as a separate species from. T. tenellus
This
is the other trans-Andean taxon, and it is sister to tenellus as one
would predict on biogeographic grounds.
If someone does not endorse tenellus as separate from the rufus
group, then also endorsing cupreicauda as a separate species from either of those
would be unlikely and would require special explanations. The vocal differences from other taxa are
given as follows:
“Song: Compared to T. tenellus,
the song has more notes per phrase, shorter note durations and generally lower
note frequencies. It also has more notes per phrase, shorter note durations but
a longer pause after introductory note, and generally higher note frequencies,
especially for the introductory note, than in T. rufus subspecies.
Compared to T. chrysochloros, the song has a slower pace, longer pause
following the introductory note, generally longer note durations and generally
lower note frequencies.”
In
their Factor Analysis (Fig. 4A), cupreicauda shows no overlap with the
other taxa on the axis heavily weighted by pace (slow) and note length (short).
In
terms of phenotype, the most striking feature to me is that the tail color is
closer to distant chrysochloros than to any other taxon, and in fact, it
differs the most in this feature from parapatric tenellus. The yellow eyering distinguishes it from all
other taxa. See p. 28 for a listing and
discussion of other color differences.
C.
Recognize T. muriciensis as a species.
As
rightfully emphasized by Dickens et al., little comparative material was available:
“Diagnosis: We had
little material available for the diagnosis of the new species Trogon
muriciensis, particularly regarding external morphology, so caution must be
taken until more information is collected. For comparison of plumage coloration
and barred patterning, only the holotype was available. For morphometric
traits, in addition to the holotype, we had measurements from the paratype and
a ringed individual. For other discrete traits, we had photos from online
depositories, in addition to those of the holotype (Supporting Information, Fig. S8) and ringed individual. For the song, we had slightly
more material, with recordings from five separate individuals (including the
holotype).”
The
vocal differences from other taxa are given as follows:
“Song: Compared to T.
chrysochloros, the song of T. muriciensis has fewer notes per
phrase, slower pace, longer note durations, longer pause following introductory
note and generally lower note frequencies. It is similar to T. r. rufus
but with generally more notes per phrase, higher introductory note frequencies
and higher loudsong note low frequencies. Compared to T. r. sulphureus,
it has wider bandwidth frequencies and generally more notes per phrase, whilst
against T. r. amazonicus, it has faster pace, shorter note durations and
a higher frequency introductory note. In relation to T. tenellus, it has
a greater number of notes per phrase, shorter pause after the introductory note
a generally lower introductory note high frequency, and generally lower peak
and high loudsong note frequencies. It differs from T. cupreicauda
by having fewer notes per phrase, longer note durations but a shorter pause
after the introductory note. The bandwidth frequencies of the introductory and
loudsong notes are generally wider than all other taxa, except T.
chrysochloros.”
In
terms of phenotype (with their caveats concerning N), there is no single
diagnostic character than I can see, but rather a combination of differences
not shared with any other taxon. See p.
30 for an enumeration of the ways it differs from adjacent chrysochloros
and members of the nominate group.
Genetically (Fig. 5), the single sample is sister to all 9 samples of chrysochloros;
however, all nine are distant, i.e. from São Paulo south (no samples from
closer Bahia to RJ), so that result would be expected on the basis of isolation
by distance alone.
D.
Treat chrysochloros as a separate species from T. rufus
The
vocal differences from other taxa are given as follows:
“Song: More notes per phrase, faster pace, shorter note durations
and pause following introductory note, as well as higher note frequencies and
wider introductory note bandwidth than T. rufus subspecies. The greater number
of notes per phrase, faster pace and shorter durations are also diagnostic
against T. tenellus. Compared to T. cupreicauda, the pace is
faster, the pause duration shorter and frequencies usually higher.”
In
their Factor Analyses (Fig. 4A, B [beware that B is mis-labeled as
Trans-Andean]), chrysochloros shows no overlap with the other taxa on
the axes heavily weighted by pace, pauses between notes, and number of notes.
As
for phenotype, this taxon has a relatively smaller bill that is more highly
serrated than any other taxon. The
density of barring on the wing panel and undertail coverts is diagnosably higher
than for any other taxon. The Diagnosis
says that it can be diagnosed from all other taxa (except evidently muriciensis)
by its blue-gray to white eyering, but I think this is an error – see tenellus. See p. 25 and the plates for additional
differences.
Discussion:
There
is no doubt that all taxa are diagnosable at some phenotypic level. But at what rank? Dickens et al. noted introgression at the
phenotypic level between T. r. rufus and T. r. amazonicus, T.
r. amazonicus and T. r. sulphureus, and T. r. rufus and T.
r. sulphureus; thus, they treated them as subspecies of the same species,
which is the logical treatment. Contact
zones are valuable test cases. Those
three contact zones provide a defensible standard for seeing which phenotypic
characters matter and which ones don’t in terms of barriers to gene flow. In the vocal analyses, these three taxa
mostly overlap in every feature analyzed, so song, as we have known empirically
for 70+ years, matters. The characters
that do not seem to matter in terms of barriers to gene flow, at least in this
group of trogons, are tail color (which varies dramatically among the three),
presence of subterminal tail band, presence of breast band (no surprise here,
because there is intraspecific variation in this in other trogons), width of
undertail barring, eye-ring color (ranges from blue to yellow even within amazonicus)
and any mensural characters. In other
words, almost every plumage and morphological characters they measured is irrelevant
as a barrier to gene flow, and so an important message of this paper is that
all these characters may be irrelevant when considering species limits in
trogons. (And I wish they had measured
the implications of this for the highly flawed Tobias-BLI 7 point scoring
scheme in terms of taxon ranking, but this would have added a tangent that
would likely have increased the paper length by a page.)
The
only other known contact zone is the one between tenellus and cupreicauda
at the Panama-Colombia border, where there are no documented cases of
introgression. Therefore, with the usual
caveats, I think we can take the differences between tenellus and cupreicauda
as potential isolating mechanisms, at least if they differ from those between
taxa in the T. rufus group. These
“if/then” comparative extrapolations come with obvious solutions, but in the
absence of alternatives, at least provide defensible rationale for
extrapolation to allopatric taxa in terms of assessing potential barriers to
gene flow, for better or worse. However,
none of the phenotypic characters other than voice show any difference from
those shown by Dickens et al. to not be potential isolating mechanisms in the T.
rufus group.
That
leaves vocalizations as the best proxy for estimating gene flow or lack of it.
I
personally am not the person to assess what the vocal differences mean in a
trogon framework -- -way too rusty. Are
the reported differences comparable to species level differences in other
trogon groups? I will leave that up to
those of you who have extensive recent comparative experience with trogon
voices. To me, when I listen to the
recordings, all I hear are the features in common, which to me are considerable;
but then again, many for-sure trogon species sound moderately similar. Is the absence of playback trials crippling
given what I perceive as subtle differences?
Ridgely & Greenfield (ergo also Mark Robbins) in the taxonomy volume
of Birds of Ecuador were consistently alert to vocal differences between bird
taxa west and east of the Andes in that country; however, they considered the
differences between voices cupreicauda and sulphureus to be only
“slight”, which is slightly worrisome to me. So, I look forward to your
comments.
A.
Treat tenellus as a separate species from T. rufus. I hesitate to make a recommendation on this one
other than noting my subjective feeling that I trust the authors of this paper on
this one because of the depth to which they have gone in these analyses. Certainly, tenellus occupies a nearly
unique multivariate space in the Factor Analysis of plumage characters in both
sexes (Fig. 2), more distinctive than any other taxon other than chrysochloros. However, in terms of vocal characters, tenellus
overlaps nearly completely with the T. rufus group despite differing in
average ways from them.
B.
Treat cupreicauda as a separate species from. T. tenellus. Because there is no phenotypic evidence of
gene flow between presumably parapatric cupreicauda and tenellus, I
regard this alone as a sufficient criterion for species rank between those two.
See the Discussion on p. 32 --- the authors were keenly aware of the
significance of this. With cupreicauda
vocalizations not overlapping with those of the other taxa in multivariate
space, we have additional indirect evidence for species rank. I’m also impressed with the phenotypic
differences between cupreicauda and tenellus, which are arguably
greater than between any two adjacent taxa.
The genetic distance between the two appears at least as larger as that
between any two adjacent taxa in the tree; however, there were no genetic
samples from Colombia, much less NW Colombia, closer to the contact zone than
the sample of cupreicauda from Provincia Esmeraldas, and perhaps sampling
within that ca. 700 km gap might produce a different result.
C.
Recognize T. muriciensis as a species. With a tiny N and no truly diagnostic
characters known, this one unfortunately represents the weakest case for
species rank, as the authors noted.
D.
Treat chrysochloros as a separate species from T. rufus. With its distinctive
plumage characters and vocalizations, the evidence for this split is strong in
my opinion.
Recommendations:
A.
Treat tenellus as a separate species from T. rufus. I’m ambivalent on this one, which is awkward,
because the evidence is nearly mandatory for treating its sister taxon cupreicauda
as a separate species, which would make Trogon rufus a paraphyletic
taxon if tenellus were included in T. rufus but not cupreicauda. On the other hand, at the population-species
level I don’t think monophyly, especially when only two labile mtDNA loci were
sampled, is a valid requirement for species rank (as I have argued several
times previously). With every passing
month, it seems that new data reveal ancient hybridization among species that
would make perilous the use of any single gene tree as representing the “true”
history.
B.
Treat cupreicauda as a separate species from T. tenellus. YES. I
think these have to be treated as separate species given the parapatry with no sign
of introgression.
C.
Recognize T. muriciensis as a species.
Ambivalent. The endangered status
of this one should not, in my opinion, influence the taxonomic decision;
otherwise, this undermines the credibility of the scientific process. Certainly this should be recognized as a
separate subspecies, minimally, and I look forward to others’ comments on this.
D.
Treat chrysochloros as a separate species from T. rufus. YES on this one for reasons given above.
E.
English names: Dickens et al. proposed the following:
• Trogon tenellus = Graceful
Black-throated Trogon
• Trogon cupreicauda = Kerr’s
Black-throated Trogon
• Trogon rufus = Amazonian
Black-throated Trogon
•
Trogon muriciensis = Alagoas
Black-throated Trogon
•
Trogon chrysochloros = Southern Black-throated Trogon
• Trogon chrysoc
A
lot of you don’t like long compound names.
In fact, Tom’s blood pressure just skyrocketed when he read this. I understand that view, but in this case I
like the compound names because: (1) it keeps intact the link to Black-throated
Trogon, thus making it clear within a long list of trogon names which species
are members of the T. rufus
superspecies; (2) maintaining Black-throated in the name retains a somewhat
useful character in separating them from other trogons within their range (I
think); (3) they are marginally distinguishable anyway by plumage; and (4) if
one drops the “Black-throated”, then we would probably have to invent new names
for two of the species because “Southern Trogon” and “Amazonian Trogon” are
misleading names from the perspective of the genus as a whole. Southern Black-throated Trogon, as noted by
Dickens et al., resurrects a historical name, thus providing continuity with
older literature.
Also,
“Graceful” is somewhat unsatisfactory for tenellus (Latin for
“delicate”, fide Jobling), with or without Black-throated, although as
noted by Dickens et al., it resurrects the historical name used in Middle
American bird literature from at least Ridgway (1911) on, until Eisenmann
(1955) changed it to Black-throated after its lump (Peters 1945) into T.
rufus. Some people don’t care about
historical continuity, but for researchers, it is helpful, e.g. “Graceful
Trogon” brings up 597 hits on Google, including Gould’s monograph on trogons,
and 13 hits in Google Scholar.
“Kerr’s”
is an eponym, which will anger the anti-eponymous zealots; however, Kerr was a
female collector, which was highly unusual for the era (1912!), an American
living in Colombia and collecting birds, and she collected the type specimen. In
my opinion, not only deserves the honor but also calls attention to her
generally overlooked contributions. See
the attached provided through
Gustavo Bravo, admirably dug out by Andrés Cuervo. (You may have lost your chance to buy
Ingram’s Milkweed Cream, unfortunately).
At
this point, for English names, let’s keep it simple: A YES means acceptance of the proposed names,
at least for those we end up recognizing, and a NO means something else, either
no compound names or modifications of the modifiers or both.
[Revision
15 June 22: To really keep things simple, let’s go Y/N on compound names vs.
simple names for now, and worry about modifiers later]
References:
DICKENS, J. K., P.-P. BRITTON, G. A. BRAVO, AND L.
F. SILVEIRA. 2021. Species limits, patterns of secondary contact
and a new species in the Trogon rufus complex (Aves:
Trogonidae). Zoological Journal Linnean
Society: 1–42.
Van
Remsen, September 2021
Comments from Areta:
“A. YES. Plumage and genetics support this
split. See comments on C regarding vocalizations.
“B.
YES. Mostly based on the apparently distinctive vocalizations (despite
methodological shortcomings), marked change in tail hue over a short distance
near the zone of geographic proximity between them and less so based on the
phylogenetic information (the single sample of cupreicauda is quite away from the southern limit of tenellus).
“C.
NO to recognizing muriciensis as a
separate species. The data is very limited and unsatisfactory to make this
move. The shallow genetic divergence, lack of diagnostic plumage features, and
similar vocalizations to chrysochloros
indicate to me, at most, subspecific status. Regarding the vocalizations, I
have trouble in seeing the most basic parameters of the spectrograms in Figure
4 (e.g., time and frequency values), but these seem to have been built using
different scales, thereby presumably distorting the similarities and
differences among vocalizations. It also seems to me that there are important
vocal differences among sexes in Trogons
(fleetingly disregarded by the authors) that were not taken into account when
making the comparisons; this can easily be heard and seen in spectrograms when
couples of birds duet or respond to each other. I have trouble in matching the
presumably "typical" songs depicted in the spectrograms to results of
the discriminant analyses: songs which look very different overlap widely,
suggesting that measurements were not able to capture key features of the
sounds or that the spectrograms are not so typical. Finally, several recordings
of chrysochloros sound exactly like muriciensis.
“D.
YES to splitting chrysochloros from rufus. Plumage, vocal and genetic data
agree in this split.”
Comments
from Donsker:
“E. My formal vote is NO. I think that we can come up
with better English names than those proposed.”
Comments from Bonaccorso:
“A. YES, but it makes more sense to accept 921B.
“B. YES. T. tenellus and T. cupreicauda seem to
have reached enough phenotypic differentiation (especially tail color; I love
the tail-hue figure!) and vocal differences. However, I am not impressed by
their genetic differences because the range of the species along Colombia was
not covered in the phylogenetic analysis, thus, differences could result from
isolation by distance. However, the possibility of parapatry and lack of
intermediate individuals supports the potential lack of gene flow.
“C. “YES, but a bit hesitantly (I agree with Nacho that the data
are sparse). Phenotypically, the Alagoas population is not so different from chrysochloros.
However, vocally (and I am no expert), it seems to be very different. Still,
the low sample size (N = 5) may not reveal the variation spectrum of
vocalizations in the Alagoas population. Genetic differentiation is weak and
based on one sample, whereas genetic sampling of chrysochloros does not
cover localities closest to the Alagoas population. In short, I think the
decision should be based on the acoustic data if some of you (experts on the
subject) think the level of differentiation merits species status.
“I disagree with the position that no special considerations
need to be made when the decision is about endangered species. I think there
has to be asymmetry when considering species that may or may not be endangered.
If, in the end, they are no good species, some money and effort may be lost,
but at least the habitat of the species may get some level of protection for a
while (which, needless to say, will benefit many other species). It is much
worse to err in the other way. Not recognizing an endangered species and waiting
for more data may have much worse consequences, especially in cases like this,
where the range seems tiny. Also, from my experience with the Blue-throated Hillstar,
the fact that we recognize new, endangered species does not guarantee immediate
incorporation into IUCN lists (even after repeated requests to BirdLife
International). Meanwhile, deforestation continues, and funds are not available
for research, research-based conservation, or protection. So, our delay in
recognizing these species may have fatal consequences under the current
circumstances.”
“D. YES. In this case, vocal and genetic evidence seems to
support species status. I don´t think phenotypic differentiation is so strong
or useful in defining species status in this case. T. sulphureus seems
very different from rufus and still, they hybridize.”
Comments
from Pacheco:
“A, B and D. YES. Plumage, genetics, and vocal
repertoire provide a good endorsement for treating these taxa at the species
rank.
“C. A vacillating YES, for the exact reasons explained by Elisa.
Obtaining more data (vocalizations, genetics) and conservation measures for
this population becomes dramatic races against the clock.”
Comments
from Stiles:
“YES to all except C.”
Comments
from Josh Beck on Part E (voting for Claramunt on English names): “I have witnessed a lot of mild confusion among both friends
and strangers in terms of which Trogon species carries which name, as so many
of the names could readily apply to multiple species. I also at times have to
stop and think for a second which of the white-tailed Trogons is called
White-tailed, which of the green-backed is called Green-backed, and which of
the Amazonian Trogons is called Amazonian. Across all this, despite vocal
differences between the potential daughter species, Black-throated is a name
that no one confuses as it corresponds to one of the more visually and vocally
distinct Trogons (seas currently defined). For this reason, despite the
proposed compound names being awkward, I am highly in favor of retaining
Black-throated in the name. Coining 5 novel names in a group of birds that all
look very similar might make for more elegant titles in field guides but will
not serve birders or other English name users, and I really think that “will
these names best serve common name users” needs to be a driving principle. As
far as the modifiers, I feel that Graceful is not very helpful as it provides
zero identification / location information. I would suggest Central American or
Middle American is an obvious possibility that is far more useful. As for
Kerr’s, as deserving as Kerr may or may not be, and independent of one’s views
of patronyms, the name provides no information or help locating or identifying
the species. Choco seems, at first glance, the obvious choice for a far more
useful name. This situation is analogous, to me, to the names for the
Daggerbills. Within the two, fortunately one of them bears a useful geographic
modifier so it is easy to keep them straight, as Geoffroy’s doesn’t really tell
the average birder or English speaker anything. If they hypothetically were
named Graceful Daggerbill and Geoffroy’s Daggerbill there would be a lot of
people, myself included, frequently pausing for a moment to remember which one
is which.”
Comments
from Jaramillo:
“YES to A, B, C, D. I guess it would be good to give a set of opinions
and viewpoints, but they have all been made by others so far. I do think I am
giving benefit of the doubt to the researchers who put this together in
painstaking detail, and have provided us a framework. In a sense, I do not feel
qualified to counter their arguments on these opinions.
“E
– NO. The compound names do not do much here that is beneficial in my mind. If
we are to have a compound name, it would be better for it to be “yellow-bellied
trogon” to separate from the red-bellied species, I am not serious, but in the
end that would be more useful to English speakers. At this point, with such a
massive split, the name “Black-throated Trogon” seems to be destined to be
forgotten and in this case, likely that is a good thing given that none of
these forms are best served by that name?”
Comments
from Lane:
“A. YES.
“B. YES.
“C. No. The
characters distinguishing its voice from T. chrysochloros seem weak, and
weakened even more by the recording on X-c recorded in 2021 <https://xeno-canto.org/628115> that
has a song with more notes. I just don't think this taxon will have significant
defining characters once it is given more scrutiny.
“D. YES.
“E. NO. I
am not all that in favor the "XXX Black-throated Trogon" because I am
not usually very impressed by compounded names of this nature, particularly
when there are so many syllables involved (kind of a "Black-backed
Three-toed Woodpecker" sort of situation in my mind).
"Black-throated" is not at all a defining character for any member of
the complex, so it is a name I'm happy to lay aside in preference for something
like "Graceful Trogon" (T. tenellus), "Kerr's
Trogon" (T. cupreicauda), "Sulphur-bellied Trogon" (T.
rufus and related taxa), and "Saffron-bellied Trogon" (T.
chrysochloros incl. muriciensis)... or something along those lines.”
Comments
from Nigel Collar (voting for Bonaccorso on English names):
“E.
YES for compound names, although I’d go for:
Northern Black-throated Trogon T.
tenellus
Western Black-throated Trogon T.
cupreicauda
Central Black-throated Trogon T.
rufus
Eastern Black-throated Trogon T.
muriciensis
Southern Black-throated Trogon T.
chrysochloros
“Reasons: Just keeping to the points of the compass (plus
Central—admittedly the hardest to stomach) homogenizes everything and makes it
seem much fairer (more equal) and simpler; they all get two extra syllables
with the emphasis on the first—slips off the tongue. It doesn’t matter to me
that the Northern is more western that Western. Everyone’ll get used to it
quick enough.
“(Personally I don’t buy muriciensis as a species, and I’d
treat it in Southern. (You could almost dare to call Central “Eastern” in that
case, but let’s not go there…)”
Additional
comments from Josh Beck (voting for Claramunt on English names):
E.
[YES for compound names].”I've mulled this over quite
a bit and asked around a wide range of Neotropical birders and guides for their
opinions, so this is my vote but based heavily on the opinions of a range of
American (US), South American, and European birders and guides. I do think that
Dan and Alvaro make good points about the lack of uniqueness or elegance of the
compound names. I also dislike them. Really, there are almost no good names in
the Trogons. But new novel names coined will be equally useless and have
no history, further disrupting stability and losing the affinity of these 4-5
species. The daughter species here are a phylogenetic group and
"black-throated" is one of the better understood/better known names
among a sea of not terribly informative nor memorable Trogon names. Removing
the black-throated part would be acceptable if new, useful, elegant, uniquely
identifying names were available, but barring divine inspiration, any and all
names for these birds are going to fail to be uniquely identifying or inspiring
or memorable. For that reason, I really think, and all but one of the people I
asked agreed, that despite the ugliness and awkwardness, it's far more useful
to retain the black-throated descriptor. For what it is worth, most people I
asked also thought that the modifiers to "Black-throated Trogon"
should be geographically based as anything else is, again, not really helpful
in distinguishing the daughter species nor remembering the names.”
Comments from Remsen: YES to all except C, for which
the evidence for species rank is weak, as noted in the proposal and several
comments. As for E (compound names), I
find Josh’s comments persuasive. If
someone had come up with a compelling set of one-word “first names”, then that
would be one thing, but in the absence of this, I think the compound names are
more useful in that they help sort out a bunch of generally poor trogon names
by branding one species complex with a name that provides historical
continuity.”
Comments from Whitney (voting for Robbins/Schulenberg on E):
“E. I favor retaining “black-throated” as part of the English
names. My name is Bret, and (mostly in
Latin America) I am occasionally asked if it’s a nickname, or, what Bret means.
Heck, I have no idea, so I tell them,
“It’s meaningless, but my mom gave it to me, and it’s stuck.” I expect this is exactly what would happen
with any of the names proposed by Dickens et al. I think the names they proposed are fine. “Graceful” may leave some wringing their
hands, but heck again, it doesn't matter, because it will serve the intended
purpose of labeling a geographically defined entity (an entity that has borne
that name in the past). Now, if we
remove “black-throated” from the names, we do, in fact, lose valuable
information, as the "black-throated trogon” (T. rufus complex) everywhere
is distinguished from all other (especially sympatric) trogons in being a
lowland, terra firme understory (~3-9 m), yellow-bellied, barred-tailed,
eyering-lacking, largely insectivorous Trogon (you notice I didn’t
mention “black-throated” because that feature is truly useless — but again,
that’s beside the point, it’s just a name, and a name that’s worked well
forever). It is useful to ornithologists
and birders to retain “black-throated” because it immediately categorizes these
birds across their wide geographic radiation, and it provides a logical (and
phylogenetically consistent) order among the multiple species of trogons
sharing the range.
“I think it’s important to recognize that compound naming was not
applied in the T. viridis split, which has led to confusion that would
have been avoided by retaining “white-tailed”. I think it would make sense to revisit those
names, and to apply compound names to future splits/descriptions of trogons
(and, imho, most other splits, such as was done with the Hypocnemis cantator
complex, which are now all “warbling-antbirds”… which, yes, don’t warble… but
no one really cares [I don’t think…]).
“As to the status of T. muriciensis, I would vote NO from a
purely scientific perspective, no emotion in the mix. I think there is a strong likelihood that
having an appropriate geographic sample of tissues from the northern end of the
range of chrysochloros would further close, but not erase, the
relatively minor genetic gap with the single sample from Alagoas. At this point, there are multiple species
restricted to the "Pernambuco Center of Endemism”, including a highly
distinctive curassow that has just recently been reintroduced there (project in
very early stages). These multiple
species and subspecies, and especially the forests where they occur, are
clearly “on the map” for Brazilian and international conservation priorities,
and I believe the Alagoas/Pernambuco region was the focus for conservation
funding raised at a recent Global Birdfair in the UK. Even if the region were not receiving much
global attention, I would not recommend species status for the Alagoas bird —
given its very close similarity to chrysochloros — without a more robust
analysis.”
Comments from Robbins: “Although the authors have gone
into extraordinary detail from a plumage, soft part, and vocal standpoint, the
genetic data are weak and in the case of proposal B, potentially suffer from
the lack of sampling. I would prefer to
have more genetic data before making a definitive recommendation; however, that
might not be forthcoming for some time. The
reason I put emphasis on that data set is that there are not consistent
differences in morphology and vocalizations across this complex. It should go
without saying that differences in plumage among most of these taxa are
relatively small, with male tenellus and rufus sulphureus
standing out in dorsal tail coloration. Moreover, much of those dorsal tail
differences are represented within the Amazonian populations, i.e., amazonicus,
nominate, and sulphureus. Thus,
if one treats Amazonian populations as a single species then dorsal tail
pattern becomes questionable as a species-defining character.
“A) NO, because of the above concerns with the genetic data
coupled with overlap between tenellus and the Amazonian group of rufus
in vocalizations. Also, see comments under B.
“B) NO, we need data where tenellus, at least
theoretically, comes into contact with cupreicauda at the
Panamanian/Colombian border. Note that
there is only a single genetic sample of cupreicauda, and it is from
Esmeraldas, far from the potential contact zone. That interface might shed
light on the importance of dorsal tail pattern (given the differences between
these two) and the importance of vocalizations. Depending on those data, I could see where tenellus/cupreicauda
are treated as a species and it is considered a separate species from
cis-Andean populations.
“C) NO, data are too limited to assess taxonomic status.
“D) NO, although given current data, I lean more towards
recognizing this as a species, primarily because of differences in
vocalizations as outlined in Fig. 4 in Dickens et al. and the disjunct
distribution. However, to be consistent with what I have outlined above I vote
No. Note that plumage and perhaps soft
part colors are not unique to chrysochloros, and the limited genetic
data indicate that it is close to Amazonian populations (no surprise).”
Comments from Zimmer:
“A. YES. Despite lack
of diagnostic vocal differences, I have a hard time accepting that there are
two conspecific taxa whose ranges are widely disjunct, and separated not only
by the Andes, but by the presence of another taxon in the same group (cupreicauda), that is demonstrably
different from both the trans-Andean taxon and the cis-Andean taxon, and that cupreicauda is parapatric with tenellus without evidence of
intergradation between the two.
“B.
YES. Although it is not listed as a
subset of this Proposal, it would also follow that I would also vote “YES” on
treating cupreicauda as a separate
species from rufus/amazonicus/sulphureus. As Van noted (in the Proposal),
phenotypic/plumage characters are all over the map in “Black-throated Trogon” (sensu lato), and even within some
subspecies groups (the Amazonian/Guiana rufus-group),
and really don’t seem to work as isolating mechanisms. Given that, to quote Van “That leaves
vocalizations as the best proxy for estimating gene flow or lack of it.” Accordingly, to my ears, songs of cupreicauda are distinct from those of tenellus to the north and west, and
those of the various Guianan/Amazonian cis-Andea forms to the east and
south. The absence of phenotypic
evidence of gene flow between these two parapatric populations is further
supporting evidence, which, admittedly, is weakened somewhat by the lack of
sampling from closer to the potential contact zone.”
“C.
NO, at least not for now. Sample sizes
are just too small in my opinion, particularly given the absence of data from chrysochloros from Bahia to Rio de
Janeiro, more proximate locales, which, when added to the mix, could well
narrow any apparent gaps regarding potential differences in vocalizations,
plumage, and genetics. I would note,
however, that from the vocal samples I’ve listened to, muriciensis is closer (at least in song characters) to Amazonian
populations than to fellow Atlantic Forest taxon chrysochloros. This would
certainly fit an established pattern of several Atlantic Forest taxa from the
Alagoas-Pernambuco center of endemism having their closest relatives
distributed in SE Amazonian rather than the S Atlantic Forest. For example, think Automolus lammi (closer vocally and genetically to A. paraensis than to A. leucophthalmus), or Thamnophilus aethiops distans, or Hemitriccus griseipectus naumburgae. There’s also the broader pattern of
widespread Amazonian taxa having highly isolated but closely related
populations in the northern part of Brazil’s Atlantic Forest (south through
Espírito Santo to N Rio de Janeiro for many of them) – think Cinereous
Antshrike, Cinereous Mourner, Ringed Woodpecker, Bright-rumped Attila, Thrush-like
Wren, White-winged Potoo, etc.
Obviously, there are other species-pairs where the close affinities are
between Pernambuco regional specialties and SE Atlantic Forest birds (Myrmotherula snowi/unicolor comes
rapidly to mind), but the point is, that there are numerous examples of taxa
from the forest fragments of Alagoas/Pernambuco being more closely related to
Amazonian counterparts than to any taxa from farther south in the Atlantic
Forest. So, it’s not implausible to me
that muriciensis could be distinct
from chrysochloros, but I do think we
need more data points to be confident that is the case.”
“D.
YES. This one is the most different
vocally (perhaps, along with cupreicauda)
from any of the others, and those vocal differences are congruent with
phenotypic distinctions (even if it turns out these aren’t important as
isolating mechanisms), ecological differences, genetic differences, and
established biogeographic patterns.”