Proposal (923) to South
American Classification Committee
Treat Haplophaedia aurelia
and Haplophaedia assimilis as conspecific.
This is a reworking of a proposal Pam Rasmussen has submitted to
NACC. Because this issue is barely with
the aegis of NACC, we have asked Pam if we could reverse-engineer the proposal
and submit it to SACC as a proposal to lump the two species, because I think
SACC should take the lead on this one.
As you can see from Pam’s proposal, the main reason she favored a split
was just because the issue was of borderline concern to NACC and she thought
NACC should, therefore, follow the recent split. As you can see from Pam’s proposal, going
strictly by Schuchmann et al. (2000), there really isn’t much to support the
split The reason SACC treats them as separate
species is because our baseline classification started with Dickinson (2003:
H&M3), which followed Schuchmann, and we have had a “SACC proposal needed”
notation in the footnote from our first online classification:
“61. Haplophaedia assimilis was formerly
(e.g., Peters 1945, Meyer de Schauensee 1970) considered a subspecies of H.
aureliae, but Schuchmann et al. (2000) provided rationale for treating
it as a separate species, representing a return to the classification of Cory
(1918). Proposal needed (to assess validity of
this split). The Ecuadorian subspecies russata
was also formerly (e.g., Cory 1918) considered a
separate species from Haplophaedia aureliae, but Peters (1945)
treated them as conspecific.”
Tangentially, one reason for maintaining these two as separate
species is in the context of continued recognition of Haplophaedia lugens,
the Western Andes representative of this complex, as a species. Although aware that it was the most
distinctive taxon in the complex, Zimmer (1951) still treated lugens as
the west slope subspecies of Haplophaedia aureliae, as noted in our SACC
note:
“61a. Zimmer (1951b) provided rationale
for treatment of H. lugens as a subspecies of Haplophaedia aureliae,
but this has not been followed by subsequent authors; see Schuchmann et al.
(2000), who used specimen locality evidence to indicate local sympatry.”
Zimmer’s treatment actually makes more sense in some ways, but
Schuchmann et al. (2002) found specimen evidence that they interpreted as
indicating sympatry:
“In agreement with more recent
taxonomic works (Wetmore 1968, Sibley & Monroe 1990), we confirm the
species status of H. lugens. Zimmer (1951) doubted the specific validity
of the taxon, not only for morphological reasons but also because of assumed
allopatry with H. a. russata, and questioned specimens from the eastern slope
(Papallacta) in Ecuador. However, our results not only support this record and
another adjacent collecting site (Baeza), but give further evidence for the
sympatry of these taxa (for several russata specimens from northern
central Ecuador, see Appendix). The high plateau of Porculla (below 3000 m)
probably serves as a pathway for cis- and trans-Andean invading ….. “
I hope someone with strong knowledge of the history of specimen
localities can investigate the evidence for sympatry, including considering the
possibility of pseudo-sympatry created by wandering individuals. But for now, let’s assume that that true
sympatry occurs between lugens and aureliae, and hereafter focus
on aureliae-assimilis.
Given
the overall morphological and plumage similarities (see illustrations in Pam’s
proposal), at the outset, burden-of-proof would seem to fall on treating assimilis
and aureliae as separate species.
In our opinion, that burden-of-proof was inadequately established by
Schuchmann et al. Here is their
rationale:
“This treatment is justified not
only by their complete geographical isolation from H. aureliae
and H. lugens but also by the distinct, relatively apomorphic
coloration pattern (puff colour, very dull green plumage). Nevertheless, we
think that further field studies are needed to verify whether H. assimilis
can also be ethologically distinguished from its sister taxa (e.g., song
structure, display).”
As
for Schuchmann et al.’s reasoning, disjunct distributions are not considered
evidence for species rank. Further, the differences in leg puff color are
step-clinal, with a major part of the distribution of occupied by taxa that are
intermediate in leg puff color, bridging the pure white northern birds and the
buff southern birds; also, Plate 2 in Schuchmann et al. illustrates male H.
a. galindoi from the Darién as having part white and part tawny leg puffs,
as in the distant populations from Ecuador.
But then here is the NACC proposal by Pam Rasmussen:
______________________________________________________________________________
2022-A-6 N&MA
Classification Committee pp.
Split Haplophaedia
assimilis from Greenish Puffleg H. aureliae
Description of the problem:
Within the NACC region the Greenish Puffleg Haplophaedia
aureliae occurs only on a few mountains in eastern Panama (cerros Pirre,
Malí, and Tacarcuna), but has long been considered widely distributed on Andean
slopes from Colombia south to northern Bolivia (e.g., Wolters 1975‒1982, Sibley
and Monroe 1990, AOS 1998, Schulenberg et al. 2007). Numerous earlier sources
(e.g., Simon 1921:188, Peters 1945) have treated H. aureliae as a single
species, presumably leading to the NACC treatment. However, since its inception
SACC has treated what we treat as H. aureliae as two species, Greenish
Puffleg H. aureliae from eastern Panama at least through southern
Ecuador, and Buff-thighed Puffleg H. assimilis of the eastern Andes of
Peru and Bolivia. This was largely based on a comprehensive morphological
analysis of the genus Haplophaedia by Schuchmann et al. (2000, https://www.zobodat.at/pdf/Anzeiger-Ornith-Ges-Bayerns_39_1_0017-0042.pdf), in which they advocated reinstatement of species
status for H. assimilis, which was treated as Vestipedes assimilis
by Cory (1919; Haplophaedia not being introduced until that same year).
In addition to treating assimilis as specifically distinct, Cory (1919)
also treated floccus, russata, and lugens as full species;
the first two of these have long been subsumed under H. aureliae by
subsequent authors and the latter is generally considered specifically distinct.
The two subspecies in the NACC region, galindoi of Cerro Pirre (in c
Darien) and floccus of Cerro Tacarcuna and its spur Cerro Malí (e
Darien) and adjacent Colombia, have been subsumed within subspecies caucensis
by some (including HBW, the accounts by Heynen 1999a, b), but both were
reinstated in the HBW/BLI checklist (del Hoyo and Collar 2014).
Although Schuchmann et al. (2000) advocated specific
status for both assimilis and lugens, their case for considering assimilis
specifically distinct rested on their disjunct distribution, the all-buffy leg
puffs of assimilis vs white or bicolored puffs in aureliae, and
notably duller plumage than in aureliae (the latter described difference
not being well shown in the illustrations accompanying the paper, nor in del
Hoyo and Collar 2014). Nevertheless, Haplophaedia assimilis is also now
recognized as specifically distinct by Dickinson (2003), Dickinson and Remsen
(2013), Gill and Wright (2006), Gill et al. (2021), Clements et al. (2021), and
HBW/BLI, in del Hoyo and Collar (2016). Schulenberg et al. (2007), however,
illustrated a white-puffed bird and did not mention assimilis or that
(at least most; see below) Peruvian birds are buffy-puffed. Thus, NACC is
nearly alone among major current lists in not recognizing H. assimilis
as specifically distinct.
New information:
There does not appear to be significant new
information bearing on the split of H. assimilis, which has been
universally accepted among the four major global checklists as well as SACC. As
far as I can determine, H. assimilis has not been sequenced (though H.
aureliae and H. lugens have, and are moderately diverged; McGuire et
al. 2014). However, on the SACC list (https://www.museum.lsu.edu/~Remsen/SACCBaseline03.htm), the need for a proposal to
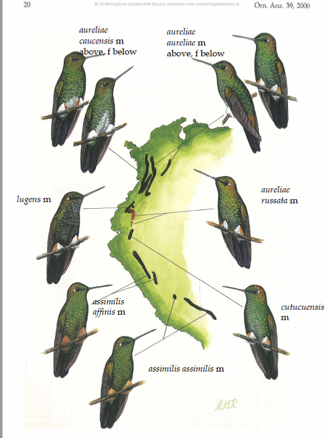
Screenshot of Plate 1 in Schuchmann et al. (2000),
with labels added.
assess the validity of this split is mentioned. In
the absence of any formal analysis, it appears that the two-species treatment
is primarily based on two plumage characters, buff puffs (differing in tone between the two subspecies)
and overall duller plumage color that differentiate assimilis from the
various forms of H. aureliae.
The following photos (thanks, Oscar!) from LSUMNZ,
however, complicate the picture, and do not seem to support a major phenotypic
break between aureliae and assimilis in accordance with the
ranges in Schuchmann et al. (2000). Taxa are from south (left) to north (right)
in both, except for lugens at the ends:


Rather than supporting the distributions of
morphological characters and thus taxa outlined by Schuchmann et al. (2000),
the most striking difference among the series (other than the distinctive lugens,
generally considered a separate species) seems to be between H. assimilis
assimilis (the left two, with the buffy puffs and lack of white scaling
below) and the two birds identified as H. assimilis affinis (from the
north of the range illustrated in Schuchmann et al.’s plate 1, the outlying
Alto Mayo of San Martín), with white puffs and strong scaling, not matching
either the plate or description in Schuchmann et al. (2000), especially as affinis
is illustrated there as having the most rufescent puffs. Also, as Oscar noted,
the cutucuensis specimen (which is from the southern end of the range of
any aureliae taxon and the next one to the north of H. assimilis
affinis), seems indistinguishable from the two affinis. In response
to my puzzlement, Oscar photographed the entire LSU series (below), which show
all the northern Peruvian series of “affinis” (upper row) to be
white-puffed and heavily scaled, unlike all the southern Peruvian and Bolivian assimilis
(lower row).

In the Acknowledgments, Schuchmann et al. (2000)
listed the museums at which they examined the 149 specimens used in the study,
and LSU is not among them, but they did list and map an examined specimen (from
the type locality; Peters 1945) from Ray-Urmaña (-6.47, -77.35) and another
from nearby Chirimoto (-6.517, -77.4), both in Amazonas, which presumably were
typical affinis. Perhaps the LSU series of “affinis” are actually
cutucuensis, and the break between H. aureliae cutucuensis and H.
assimilis affinis is farther south than shown in Schuchmann et al.’s (2000)
map? That would seem to suggest a
parapatric or possibly even sympatric distribution in this region. The
seemingly strong difference (wherever it is) between white-puffed and -scaled cutucuensis
and rufous-puffed affinis does not seem to indicate clinality. Whichever
is the case, clearly further study is needed in this complex, which
nevertheless does not necessarily support the NACC single-species position (a
holdover from pre-2000 treatments).
Effect on AOS-CLC area:
If we decide to follow
the SACC treatment and that of global lists, the
impacts on the NACC-area checklist would be simply a minor modification of the
Distribution statement, substituting “southern Ecuador” or as suggested by the
LSU Alto Mayo area specimens, “far northern Peru” for “northern Bolivia”, and
also including a Notes statement regarding the split.
Recommendation:
Given that all major global lists and SACC have been
following the Schuchmann et al. (2000) treatment for some two decades, and that
there does not appear to be any published information that refutes it, I
recommend following these sources for purposes of consolidation and stability,
at least until and if evidence accumulates to the contrary. It seems likely
that the distributional ranges of H. aureliae cutucuensis and H.
assimilis affinis (and thus the southern and northern limits of the two
species, respectively) may need to be modified, but this is a matter for a more
in-depth study and for SACC, as would be the preparation of a new proposal to
lump H. assimilis with H. aureliae if needed. Since the only
changes to the NACC region check-list will be very minor, the benefits of
following the prevailing treatment would seem to outweigh the risk of further
change.
Acknowledgements:
Many thanks to Oscar Johnson for the photos, and to
Gary Stiles for advice on an earlier draft.
Literature cited:
American
Ornithologists’ Union (1998). Check-list of North American Birds. 7th
Edition. American Ornithologists’ Union, Washington, D.C.
Clements, J. F., T. S. Schulenberg, M. J. Iliff, S. M. Billerman, T. A.
Fredericks, J. A. Gerbracht, D. Lepage, B. L. Sullivan, and C. L. Wood (2021).
The eBird/Clements checklist of Birds of the World: v2021. Downloaded
from https://www.birds.cornell.edu/clementschecklist/download/
Cory, C. B.
(1919). Catalogue of birds of the Americas and the adjacent islands in Field
Museum of Natural History. Field Museum of Natural History Publication 203,
Zoological Series, vol. 13, Part II, No. 2., Chicago, Illinois.
del Hoyo, J. and N. J. Collar (2014). HBW and BirdLife International
illustrated checklist of the birds of the world. Volume 1 Non-passerines. Lynx
Edicions, Barcelona.
Dickinson, E. C. (2003). The Howard and
Moore Complete Checklist of the Birds of the World. Revised and enlarged 3rd
edition. Christopher Helm, London.
Dickinson, E. C.,
and J. V. Remsen, Jr. (Editors) (2013). The Howard and Moore Complete Checklist
of the Birds of the World. 4th edition. Volume One. Non-passerines.
Aves Press Ltd., Eastbourne, UK.
Fjeldså, J. and N.
Krabbe (1990) Birds of the high Andes. Zoological Museum, University of Copenhagen,
and Apollo Books, Svendborg, Denmark.
Gill,
F, D Donsker, and P Rasmussen (Eds) 2021. IOC World Bird List (v 11.2). Doi 10.14344/IOC.ML.11.2. http://www.worldbirdnames.org/
Gill, F. B. and M. Wright (2006). Birds of the world. Recommended English
names. Princeton University Press, Princeton, N.J.
Heynen, I. (1999a). Greenish Puffleg. Pp. 643 in: del Hoyo, J., Elliott,
A. and Sargatal, J. (eds) (1999). Handbook of the birds of the world. Volume 5.
Barn-owls to Hummingbirds. Lynx Edicions, Barcelona.
Heynen, I. (1999b). Buff-thighed Puffleg. Pp. 643 in: del Hoyo, J.,
Elliott, A. and Sargatal, J. (eds) (1999). Handbook of the birds of the world.
Volume 5. Barn-owls to Hummingbirds. Lynx Edicions, Barcelona.
McGuire, J. A., C. C. Witt, J. V. Remsen, Jr., A. Corl, D. L. Rabosky, D.
L. Altshuler and R. Dudley. Molecular phylogenetics and the diversification of
hummingbirds. Current Biology 24(8): 910‒916.
Peters,
J. L. (1945). Check-list of the Birds
of the World. Volume 5. Harvard University Press, Cambridge, MA.
Schuchmann, K.-L., A.-A. Weller, and I. Heynen (2000). Biogeography and
taxonomy of the Andean hummingbird genus Haplophaedia Simon (Aves:
Trochilidae), with the description of a new subspecies from southern Ecuador.
Ornithologischer Anzeiger 39: 17‒42.
Schulenberg, T. S., D. E. Stotz, D. F. Lane, J. P.
O’Neill, and T. A. Parker III (2007). Birds of Peru. Princeton University
Press, Princeton and Oxford.
Sibley, C. G. and B. L. Monroe, Jr. (1990).
Distribution and taxonomy of birds of the world. Yale University Press, New
Haven and London.
Sibley, C. G. and B. L. Monroe, Jr. (1993). A world
checklist of birds. Yale University Press, New Haven and London.
Simon,
E. L. (1921). Histoire
naturelle des Trochilidae (synopsis and catalogue). Encyclopédie Roret, L.
Mulo, Paris.
Wolters, H. E. (1987‒1982) Die Vogelarten der Erde. Paul Parey, Hamburg and Berlin.
Submitted by: Pamela C. Rasmussen,
Michigan
State University
Date of proposal: 7 October 2021
_______________________________________________________________________________
When
assembling information in preparation of revising the Birds of Peru in 2009,
Dan Lane discovered that the Lima Museum (MUSM) had three specimens of Haplophaedia
“aureliae,” all from San Martín dept: 2 specimens from the Alto Mayo (collected
in 2002 on the same expedition as the LSUMZ specimens depicted above) and one
from near “Pataz” in the far SW corner of San Martin dept (about 225 km from
the Alto Mayo locality). The former two, in agreement with the LSUMZ series,
were white-tufted and had extensive white scaling below. The latter, however,
was buff-tufted and lacked scaling. After sharing this finding with lead author
Tom Schulenberg, it was clear that the two taxa must turn over somewhere
between the Mayo and Huayabamba drainages (the latter containing the type
locality for affinis) without evidence of introgression. As Pamela and
Oscar state above, this situation suggests that the two taxa are best
considered species with respect to one another.


Whereas
the LSUMZ/MUSM Alto Mayo series was collected in 2002, postdating the
publication of Schuchmann et al (2000), the single LSU specimen from Colán would
have been available during the period of the study, and documented that
white-tufted birds occurred south of the Marañon. Thus, the fact that
Schuchmann et al. did not include the LSUMZ Museum series in their study resulted
in a missed opportunity to strengthen their stance on the species limits
between the aureliae and assimilis groups.
We
further suggest that the LSUMZ specimens from Alto Mayo were incorrectly
labeled as “affinis” but represent cutucuensis instead, as Pamela
and Oscar suggest above, adding another mid-elevation east slope Andean taxon
that crosses the Marañon biogeographic barrier for a brief stretch before its
distribution ends south of it.
Discussion
and recommendation:
Schuchmann et al.’s two-species
treatment seems to be correct in this case, although their reasoning was weaker
than the specimen material before us demonstrates, and so we recommend a NO
vote on treating them as conspecific.
Van Remsen
and Dan Lane, October 2021
Comments
from Robbins:
“I vote NO for treating Haplophaedia aurelia
and assimilis as conspecific given the information presented in this
proposal that would seem to indicate that both should be treated as species.”
Comments
from Claramunt:
“NO. There is no new data that suggest that they
may be conspecific.”
Comments from Areta: “NO. The two-species treatment is more
consistent with patterns of geographic variation and morphological breaks in
this group.”
Comments from Pacheco: “NO. Considering the information
provided by Remsen and Lane, it becomes coherent to keep aurelia and assimilis
at the species level.”
Comments from Bonaccorso: “A definite NO to treat H.
aurelia as conspecific of H. assimilis, until more evidence is provided.”
Comments from Zimmer: “NO,
based upon the lack of any new data suggesting conspecificity, as well as the
fortifying evidence for maintaining the status quo provided by Van & Dan in
the Proposal.”
Comments
from Stiles:
“NO to considering these two Haplophaedia as
conspecific, given the more detailed data from specimens provided by Van et al.”
Comments
from Jaramillo:
“NO – You had me at “morphological break.” But seriously, the data better
suggests we keep two species here.”