Proposal (950) to South
American Classification Committee
Note from Remsen: This
is slightly modified version of the proposal submitted to NACC by Oscar.
Treat Chlorothraupis
frenata as a separate species from
Carmiol’s Tanager C. carmioli
Description of the problem:
Chlorothraupis carmioli (Lawrence 1868)
is a polytypic species broadly comprised of two subspecies groups; a northern
group of three subspecies (carmioli, magnirostris Griscom 1927, and lutescens Griscom 1927) found in lowland
and foothill tropical forests from Honduras to extreme northwestern Colombia,
and a South American subspecies frenata
von Berlepsch 1907 found in the eastern foothills of the Andes from southern
Colombia to central Bolivia (Hilty 2022). Although these two subspecies groups
are highly disjunct, the intervening regions are occupied by two congenerics; C. olivacea (Cassin 1860) of the
lowlands and foothills of western Colombia and northwestern Ecuador (the Chocó)
and just reaching far eastern Panama, and C.
stolzmanni (von Berlepsch & Taczanowski 1884) of the same region but
replacing olivacea at higher
elevations and not reaching Panama (Hilty 2020a,b). Within Central America, the
two southern subspecies are somewhat more yellow and larger-billed in western
Panama (magnirostris) and much more
yellow and smaller-billed in eastern Panama (lutescens), while nominate carmioli
is found from Costa Rica north (Griscom 1927). For simplicity, I’ll refer to
these three northern subspecies as the “carmioli”
group throughout this proposal.
The current species-level treatment is largely
unchanged since each of the taxa was described. The two taxa first described, C. olivacea and C. carmioli, were each
considered species by the describing authors, and most subsequent authors (e.g.
Dickinson 2003). The ranges of the two approach each other in eastern Panama
and apparently don’t show signs of hybridization. Ridgely and Gwynne (1989),
specimen data on VertNet, and occurrence records in eBird all indicate that C. olivacea
is found on the eastern Darién mountains of Cerro Sapo,
Pirre, Quía, and Jaqué, but
is replaced on Cerro Tacarcuna by C. carmioli. C. carmioli frenata was
described as a subspecies of carmioli
by von Berlepsch. von Berlepsch’s (1907) reasoning for maintaining frenata as a subspecies of carmioli is worth reproducing here in
full:
“It is a curious fact that the Chlorothraupis of South-eastern Peru has its nearest ally in a
species which, as far as we know, is restricted to the forest-region of Costa
Rica. In fact, the resemblance between Costa Rican and Peruvian examples of
this Chlorothraupis is so great that
Messrs. Sclater and Salvin have not attempted to separate them.
“In the
meantime, having (through the kindness of the Hon. W. Rothschild) had an
opportunity of comparing five adult birds, collected by Mr. Underwood in Costa
Rica, with my specimens from Marcapata, South-east Peru, collected by Mr. O.
Garlepp, I have detected some small though apparently constant characters, by
which the Peruvian birds may well be distinguished.
“In the
latter the lores and the small feathers of the frontal line near the nostrils
are yellowish (purer and brighter yellow in the younger and more
greenish-yellow in the adult specimens), while in the Costa Rican birds these
parts are of the same dark olive-green as the upper part of the head.
“Further,
the general coloration of the upper and under parts of the body of the Peruvian
birds is of a clearer and purer green, while the Costa Rican birds show a
rather more oily or brownish tint in the plumage. The alar margin and the under
wing-coverts in the Peruvian specimens are of a clearer or more a
yellowish-green colour. The tail is of a rather
brighter green or less blackish.
“As a
rule the wings and the tail in the Peruvian birds appear to be a little
shorter.”
The yellow color of the lores is the main
plumage character that separates C. olivacea
and C. carmioli, although the differences in that comparison are much more
extreme, and give the former species its English name, Lemon-spectacled
Tanager. Many authors (e.g. AOU 1983,
1998, Isler & Isler 1987) gave C.
carmioli the English name Olive
Tanager, but the NACC (following Meyer de Schauensee 1970, Dickinson 2003, and
others) changed the English name to Carmiol’s Tanager to avoid confusion with C. olivacea (Banks et al. 2008). The
SACC also adopted this change and recommended that “Olive Tanager” be
restricted to classifications that treat C.
olivacea and C. carmioli as conspecific (Remsen et al.
2022), as the former has priority and would keep a match between the English
and Latin names. However, I am unable to find any authors that treat these two
species as conspecific, although I could be overlooking older references.
Zimmer (1947) summarized the plumage differences
between the carmioli group, frenata, and olivacea better than I am able, and it appears little has been done
on morphological differences in the complex since:
“The wide separation of the range of this form [=frenata] from that of the other members of the species is curious,
especially in view of the occupation of the intervening terrain by C. olivacea and C. stolzmanni. Both of these last-mentioned forms appear to be
specifically distinct from carmioli
with which no intergradation of characters has been discovered at any point.
The three species are undoubtedly quite closely related. The pale lores of frenata might be considered as
suggesting the bright yellow lores of olivacea,
although the equally conspicuous yellow eye ring of olivacea is not similarly suggested, and the resemblance in the
color of the lores is not very striking, quite aside from the fact that olivacea and carmioli lutescens occur very near to each other in eastern
Panamá.”
Despite the plumage similarity between the two
taxa, some recent authors have elevated frenata
to the species rank (Ridgely and Greenfield 2001, Restall et al. 2006, del Hoyo
and Collar 2016). Ridgely and Greenfield (2001) treated frenata as a species based on descriptions of the voice and the
disjunct distribution, while del Hoyo and Collar (2016) did the same, with the
following reasoning:
“Often
treated as conspecific with C. carmioli,
the two being morphologically very similar, but quite easily separated by their
different vocalizations, including song; further investigation desirable.
Monotypic.”
The IOC elevated frenata to species rank, and gave C. frenata the English name Olive Tanager, and left C. carmioli with the English name of
Carmiol’s Tanager.
New information:
Barker et al. (2015) sampled the three species
of Chlorothraupis and found that olivacea and carmioli were sisters, and that the three Chlorothraupis were embedded within Habia (separate proposal needed for generic limits). The sample of carmioli was obtained from Burns (1997)
and is a specimen of frenata from San
Martín, Peru (LSUMZ B-5510). Klicka et al. (2007) recovered the same topology,
using a sample of frenata from
Ecuador. No other genetic data appear to be published for this complex, and
none from nominate carmioli or the
other Central American subspecies. Both studies were based on a few
mitochondrial and nuclear loci. A screenshot of the Barker et al. (2015)
phylogeny is below.

Van Remsen has graciously photographed a series
of specimens of magnirostris, frenata, and olivacea housed at the LSUMZ. Photos are below. In both photos, the
taxa shown are (top to bottom): magnirostris,
two olivacea, and frenata. Note the more extensive yellow
spectacles and darker coloration of olivacea,
and the slightly more yellow lores of frenata.


Although much of the early work on the complex
highlighted the minor, albeit consistent, plumage differences, the main
differences between the two clades is in vocalizations. However, no
publications have quantified these differences as far as I am aware, and this
group was not included in Boesman’s Ornithological
Notes. A detailed description of the vocalizations is given in Hilty (2022):
“Dawn
song a rapid stream of mostly short notes, some grating or wheezy, some
musical, and typically given rapidly in groups of 3–8, then abruptly switching
to another type of note, entire sequence often lasting up to several minutes;
some song sequences consist of clear whistled notes much like those of Northern
Cardinal (Cardinalis cardinalis). Can
be rather noisy when foraging, uttering variety of mostly short, high, thin
notes, including chay, a squeaky eep, a churring wrsst, and abrupt
chut, squeezed chee and metallic whit; also a slightly buzzy seeet or seee-seeee and
staccato tik in bursts when about to
fly; in alarm a scratchy nyaaah
or cheeyah.
“Songs
and other vocalizations of frenata
are rather unlike those of the Central American subspecies. In southeastern
Peru, frenata makes an excited, rapid
rolling ki’r’r’rup-ki’r’r’rup-ki’r’r’rup-ki’r’r’rup-ki’r’r’rup..., sometimes up to ca. 8 notes in the
series with squealing, frantic quality, and often repeated over and over at
short intervals. At times song more varied, with other high or squeaky notes
inserted into the long series, e.g. ki’r’r’rup-ki’r’r’rup-ki’r’r’rup-éé-kir’r’r’r-éé-kir’r’r’r, squik-Skeek-Skeek–Skeek-kir’r’r-kir’r’r...
and so on for up to 30 seconds or more; also transcribed as a grating kettup or keetup. A
somewhat more melodic song (context uncertain) is a series of several similar
notes, then a series of different notes, and so on: e.g., chow-chow-chow-chow-chi-chi-chi-chow, chow,
chow, whi-chow, whi-chow, wheeup, wheeup, wheeup, wheeup, tic-chow tic-chow, tic-chow, tic-chow, tic-chow, tic-tic-tic-tic-ch-ch-ch-ch..., for 10–25
seconds.”
The songs of the carmioli group and frenata are
clearly analogous; both are run-on series of very cardinalid-like whistled
notes. The primary difference, to my ear, between the songs of the two groups
is the much more rapid delivery (note pace) of the songs of the carmioli group. Although Hilty (2022)
mentioned that frenata gives a more
rolling “ki’r’r’rup”
song, this seems to be variable, and many (perhaps most) individuals of frenata give more clear whistled songs,
as noted by Schulenberg et al. (2007). Overall, note pace seems to be fairly
consistent across the distribution of each group.
Songs of the carmioli
group:
https://macaulaylibrary.org/asset/25644
https://macaulaylibrary.org/asset/201575871
versus these of frenata:
https://macaulaylibrary.org/asset/101818 (this one contains more “ki’r’r’rup” notes)
https://macaulaylibrary.org/asset/224539281
https://macaulaylibrary.org/asset/238023
Both taxa give a wide variety of other calls
(see text from Hilty 2022 above), but differences between the two groups seem
primarily to be a lower-pitched scolding call in frenata.
carmioli group:
https://macaulaylibrary.org/asset/165887
https://macaulaylibrary.org/asset/203938651
https://macaulaylibrary.org/asset/211144
frenata:
https://macaulaylibrary.org/asset/138819
For reference, the song of C. olivacea is more like that of frenata in terms of pace:
https://macaulaylibrary.org/asset/149272491
Effect on SACC area:
Splitting frenata
from the carmioli group would add one
new species to the SACC checklist area.
Recommendation:
I recommend a YES vote on splitting frenata from the carmioli group. C. carmioli
would retain magnirostris and lutescens as subspecies. My only
hesitation in splitting these taxa is a lack of genetic data for carmioli, lutescens, or magnirostris.
However, given the highly disjunct distribution from frenata, plus intervening congenerics, I would be very surprised if
these two groups did not show some genetic divergence. The plumage differences
are minor but consistent, and parallel with other species-level differences in
the group (albeit to a lesser degree). Most convincingly, the vocalizations are
consistently different, and seem to not vary considerably across the
distribution of each group (just in my cursory listening of recording, an
analysis is certainly needed!), despite the wide range of different
vocalizations given by these taxa.
Please vote on the following:
1) elevate frenata to species rank
If frenata
is elevated to species rank, new English names will be required. Clement’s / Birds of the World (2022) uses Carmiol’s
Tanager for the C. carmioli group and
Yellow-lored Tanager for C. carmioli
frenata. Although Carmiol’s Tanager has long been used for the combined
species, no other names have been used for the northern group and keeping
Carmiol’s would maintain a match with the species epithet. The two groups have
roughly comparable range sizes, likely a slightly larger distribution in frenata, so keeping Carmiol’s Tanager
with C. carmioli does go against
NACC/SACC guidelines. However, it does seem like an option to me in this case.
Olive Tanager has been used for C.
carmioli s.l. (see citations above) but the NACC changed the name from
Olive Tanager to Carmiol’s Tanager in 2008 specifically to avoid confusion with
C. olivacea (Lemon-spectacled Tanager),
so applying that name to C. carmioli
s.s. seems like a poor choice. The IOC, in elevating frenata to species rank, gave it the name Olive Tanager (see
above), but that, too, seems like a poor choice that only adds to the confusion
regarding the application of the name “Olive Tanager”. If Carmiol’s is
unacceptable to the committee as the English name for C. carmioli s.s., a separate proposal will be needed to address the
English name of that taxon. The namesake of carmioli
is Francisco Carmiol, a German immigrant to Costa
Rica who worked as a bird collector for the Smithsonian and collected the type
specimen of carmioli (Lawrence 1868,
Birds of the World 2022). Francisco was the son of the bird collector Julián Carmiol, for whom Vireo
carmioli is named (Birds of the World 2022), but little else appears to be
published about the two Carmiols. Alternatively,
Yellowish Tanager or Yellow-olive Tanager seem like decent options for C. carmioli s.s., would highlight the
more yellow coloration of at least some populations of the carmioli group, and would
be parallel to Yellow-lored and Lemon-spectacled Tanagers. Olive-green Tanager
is occupied by Orthogonys chloricterus.
Note from Remsen: If
the proposal passes, then we will need a separate proposal on English names,
but feel free to open the discussion on this in your Comments.
Literature Cited:
AOU. 1983.
Check-list of North American birds. 6th edition. American Ornithologists’
Union.
AOU. 1998.
Check-list of North American birds. 7th edition. American Ornithologists’
Union.
Banks, R. C., R. T. Chesser, C. Cicero, J. L. Dunn, A. W. Kratter, P. C.
Rasmussen, J. V. Remsen, Jr., J. A. Rising, and D. F. Stotz. 2008.
Forty-ninth supplement to the American Ornithologists' Union Check–list
of North American Birds. Auk 125:
758–768.
Barker, F. K, K. J. Burns, J. Klicka, S. M. Lanyon, and I. J. Lovette.
2015. New insights into New World biogeography: An integrated view from the
phylogeny of blackbirds, cardinals, sparrows, tanagers, warblers, and allies.
The Auk 132(2): 333-348. http://dx.doi.org/10.1642/AUK-14-110.1
von Berlepsch, H. G. 1907. Descriptions of new species and subspecies of
Neotropical birds. Proceedings of the IVth
International Ornithological Congress pp. 347-371.
Birds of the World. 2022. Edited by S. M. Billerman, B. K. Keeney, P. G.
Rodewald, and T. S. Schulenberg. Cornell Laboratory of Ornithology, Ithaca, NY,
USA. https://birdsoftheworld.org/bow/home
Burns, K. J. 1997. Molecular systematics of tanagers (Thraupinae):
evolution and biogeography of a diverse radiation of Neotropical birds.
Molecular Phylogenetics and Evolution 8(3): 334-348.
https://doi.org/10.1006/mpev.1997.0430
Dickinson, E. C.
(Ed.) 2003. The Howard & Moore
Complete Checklist of the Birds of the World, 3rd edition, Christopher
Helm, London.
Griscom, L. 1927. Undescribed or little-known birds from Panama.
American Museum Novitates 280: 1-19.
Hellmayr, C. E. 1935. Catalogue of birds of the Americas, part VIII.
Field Museum of Natural History Zoological Series Vol. XIII. Chicago, USA.
Hilty, S. 2020a. Lemon-spectacled Tanager (Chlorothraupis olivacea), version 1.0. In Birds of the World (J.
del Hoyo, A. Elliott, J. Sargatal, D. A. Christie, and E. de Juana, Editors).
Cornell Lab of Ornithology, Ithaca, NY, USA.
https://doi.org/10.2173/bow.lestan.01
Hilty, S. 2020b. Ochre-breasted Tanager (Chlorothraupis stolzmanni), version 1.0. In Birds of the World (J.
del Hoyo, A. Elliott, J. Sargatal, D. A. Christie, and E. de Juana, Editors).
Cornell Lab of Ornithology, Ithaca, NY, USA.
https://doi.org/10.2173/bow.ocbtan1.01
Hilty, S. 2022. Carmiol's Tanager (Chlorothraupis
carmioli), version 1.1. In Birds of the World (N. D. Sly, Editor). Cornell
Lab of Ornithology, Ithaca, NY, USA. https://doi.org/10.2173/bow.olitan1.01.1
del Hoyo, J., and N. J. Collar. 2016. HBW and BirdLife International
Illustrated Checklist of the Birds of the World. Volume 2: Passerines. Lynx
Edicions, Barcelona, Spain.
Isler, M., and P. Isler. 1987. The Tanagers, Natural History,
Distribution, and Identification. Smithsonian Institution, Washington, D.C.
Klicka, J., K. Burns, and G. M. Spellman. 2007. Defining a monophyletic
Cardinalini: A molecular perspective. Molecular Phylogenetics and Evolution 45(3):
1014-1032. https://doi.org/10.1016/j.ympev.2007.07.006
Lawrence, G. N. 1868. III.—A Catalogue of the Birds found in Costa Rica.
Annals of The Lyceum of Natural History of New York, 9: 86-149.
Meyer de Schauensee, R. 1970. A guide to the birds of South America.
Livingston Publishing Co., Wynnewood, Pennsylvania.
Peters, J. L. 1968. Check-list of birds of the world. Vol. XIV. (R. A.
Paynter, Ed.). Museum of Comparative Zoology. Cambridge, Mass.
Remsen, J. V., Jr., J. I. Areta, E. Bonaccorso, S. Claramunt, A.
Jaramillo, D. F. Lane, J. F. Pacheco, M. B. Robbins, F. G. Stiles, and K. J.
Zimmer. Version 2022. A classification of the bird species of South America.
American Ornithological Society.
http://www.museum.lsu.edu/~Remsen/SACCBaseline.htm
Restall, R., C.
Rodner, and M. Lentino. Birds of northern South America, an identification
guide. Vol. 1. Yale University Press, New Haven, CT, USA.
Ridgely, R. S.,
and J. A. Gwynne. 1989. Birds of Panama. 2nd Ed. Princeton
University Press, Princeton, NJ, USA.
Ridgely, R. S., and P. J. Greenfield. 2001. The Birds of Ecuador.
Volumes 1–2. Cornell University Press, Ithaca, NY, USA.
Ridgway, R. 1902. The birds of North and Middle America. Part II.
Bulletin of the United States National Museum. No. 50.
Schulenberg, T. S., D. F. Stotz, D. F. Lane, J. P. O’Neill, and T. A.
Parker III. 2007. Birds of Peru, revised and updated edition. Princeton
University Press, Princeton, NJ, USA.
Wetmore, A., R. F. Pasquier, and S. L. Olson. 1984. The Birds of the
Republic of Panama. Part 4. Smithsonian Miscellaneous Collections, vol. 150,
Washington, D.C.
Zimmer, J. T. 1947. Studies of Peruvian Birds No. 51. The genera Chlorothraupis, Creurgops, Eucometis,
Trichothraupis, Nemosia, Hemithraupis, and Thlypopsis, with additional notes on Piranga. American Museum Novitates 1345: 1-23.
Oscar Johnson, July 2022
March 2023 update from Oscar Johnson:
“he details on the
following study were overlooked in an earlier version of this proposal.
A recent master’s
thesis (Scott 2022), focused on the Cardinalidae, sampled all species of Chlorothraupis,
including one sample from Peru (frenata) and one from Panama (lutescens)
and sequenced 5,022 UCE loci. Concatenated and coalescent gene tree methods
both recovered the same topology, which shows Chlorothraupis carmioli
is paraphyletic. I have included the portion of the tree below that includes
the Chlorothraupis taxa. This is the summary of the “multispecies
coalescent gene trees produced by IQ-tree and summarized using ASTRAL”, which
includes branch lengths (unlike some of the other methods used in the thesis),
and branch numbers refer to posterior probabilities.
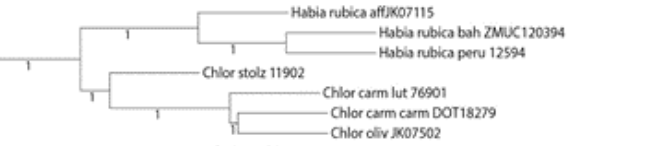
Note that “Chlor carm carm”, i.e. “carmioli”,
is mislabeled and in fact refers to frenata based on sampling locality. The
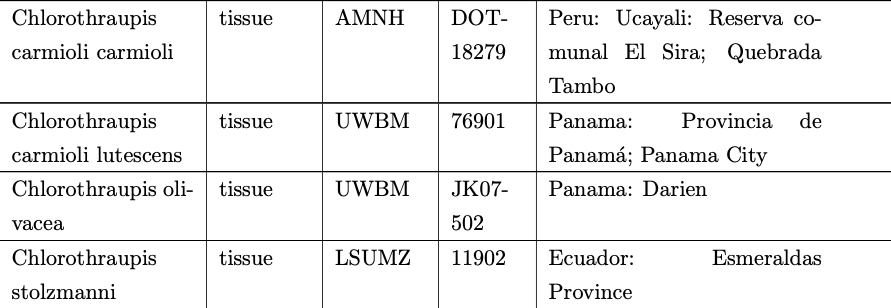
sampling table is included below for reference.
Updated recommendation:
I recommend a YES vote on splitting frenata from the carmioli group. C. carmioli
would retain magnirostris and lutescens as subspecies. The nuclear
data from Scott (2022) show that the current definition of C. carmioli
is paraphyletic, with C. c. frenata sister to C. olivacea,
and with fairly long branches separating the three groups. In addition, the
plumage differences are minor but consistent, and parallel with other
species-level differences in the group (albeit to a lesser degree). The
vocalizations are also consistently different, and seem to not vary
considerably across the distribution of each group (just in my cursory
listening of recording, an analysis is certainly needed!), despite the wide
range of different vocalizations given by these taxa.
_______________________________________________________________________________
Comments from Remsen: “YES.
Although one can always demand more data, I think the vocal differences
outlined in the proposal are sufficient grounds for treating them as separate
species. As noted back in 1989 by
Ridgely & Tudor, the highly disjunct range is suspicious, and even then,
anecdotal information on voice suggested a potential split. We have accumulated enough recordings now
that confirm those initial findings and places burden-of-proof in my opinion on
treating them as conspecific. I think
the only valid basis for rejecting the proposal might be absence of playback
trials.
“There is another
reason for conservatively treating them as separate species. As far as I can tell, the phylogenies
published so far have not sampled both the carmioli group and the frenata
groups. In both published analyses, C.
carmioli is represented by the same sample of frenata from N. Peru,
as if that sufficiently characterizes the genome of a species with disjunct
populations separated by a thousand kilometers and on opposite sides of major
biogeographic boundaries. This is yet
another example of failing to appreciate the importance of broad taxon-sampling
in phylogenetic analysis. How do we even
know that the carmioli group is monophyletic? In fact, that two other Chlorothraupis
occupy the intervening region in similar habitats makes me wonder if there
isn’t a leap-frog pattern going on here, with either stolzmanni or olivacea
or both being more closely related to one of the carmioli groups than C.
(c.) frenata and C. c. carmioli are to each
other. Seems like a long-shot, but it is
sufficient for me to object to a conspecific treatment without genetic data
confirming the monophyly of our current C. carmioli. I’d also like to hear from Kevin Burns how
much confidence he has in that topology from 2007.
“As for the plumage
similarities, such similarities are roughly comparable to those between some
species in genera closely related to Chlorothraupis: some Habia
species, some Piranga species (e.g. hepatica and rubra,
and definitely between the eventually to-be-delimited species within hepatica),
and, most notably, the other two Chlorothraupis species, which are
treated as separate species already.
Check out Plate 32 in HBW Vol. 16 to see what I mean. Frenata and carmioli are more
similar to each other than either is to olivacea or stolzmanni,
but not by much, especially if you allow for a little Gloger’s Rule darkening
of the latter two in those more humid regions.
Take away the eyering of olivacea and presto, suddenly it no
longer stands out.
“As for English
names, “Olive Tanager” is clearly DOA because of C. olivacea and
inconsistency in what “Olive Tanager” applies to. Let’s bury that one forever. I also think we should consider changing the
last name from Tanager to Chlorothraupis.
We have an opportunity to de-Tanager another non-thraupid genus, with
Spindalis and Chlorospingus providing precedents with which the world seems
comfortable. As an English name,
Chlorothraupis is no more intractable than Chlorospingus (or Hemispingus),
although unfortunately it retains the “thraupis” part
that still connotes a tanager. Although
their close relatives in the Cardinalidae, Habia and Piranga,
will likely retain “Tanager” in their name forever, at least using
Chlorothraupis will help remind us of the family-level change (gee, thanks,
Kevin). None of the four species occurs
in an English-first country. and none is a particular familiar widespread bird,
so this minimizes the impact of the instability caused by such a change.”
Comments
from Robbins:
“I vote YES for recognizing Chlorothraupis frenata
as a species. Although it would be more
informative to have a complete genetic data set for all taxa in Chlorothraupis,
the combination of the disjunct distribution (with intervening species), vocal
differences, and consistent plumage characters (in a complex where plumage
morphology is highly conserved) supports recognition of frenata as a
species.”
Comments from Claramunt: “NO. The plumage differences are
so subtle and vocalizations so varied and complex that this proposal requires a
full analysis of geographic variation examining potential clines and
intergradation. Genetic data for at least the two major groups would be
desirable too.”
Comments
from Areta:
“NO. I would normally vote unambiguously against splits based on these type of
data, but this case seems peculiar in that two Chlorothraupis species are geographically sandwiched by the two
subspecies groups of C. carmioli,
which differ subtly but diagnosably in plumage and (apparently) also in
vocalizations (mostly in the speed of delivery of the songs and in the pitch of
the scold [it also may be longer and more burry, with longer time between
inflections in frenata]). As often is
the case, plumage differences have been well substantiated, whereas differences
in the vocalizations have been cursorily described without rigorous analyses
(as underscored by Oscar). The southern C.
carmioli frenata has itself an apparently fragmented distribution that
extends across several well-known biogeographic breaks. All the evidence points
towards species status of frenata.
However, rigorous published analyses based on comprehensive datasets are
lacking, and given the structural similarities in the songs and calls and the
reduced plumage differences, one may argue that the widely allopatric frenata and carmioli groups are recently diverged. I will therefore vote NO
until the evidence is properly analyzed and put in perspective, with thorough
characterizations of the vocalizations and, hopefully, also with genetic data.”
Comments
from Stiles:
“YES for recognizing C. frenata as a species separate from C.
carmioli; this is supported by both vocalizations and plumage, albeit in
the latter, the difference is more subtle. In any case, given the existence of
two species in the long Interval between carmioli and frenata, neither
of which are known to undertake extensive migrations, I find the suggestion of
an independent colonization of carmioli so far to the south to be
decidedly far-fetched. Of the two intervening species, I am moderately familiar
with stolzmanni, which is distinctive in its more buffy-brownish color
below and its exceedingly raucous vocalizations, as well as its grayish iris
(in the 3 specimens I have collected) and agree that those of olivacea definitely
sound different from those of carmioli, which I have heard often in
Costa Rica.”
Comments
from Lane:
“YES. I am swayed by Oscar's arguments here. The
voices of the two groups (carmioli and frenata) are not
super-distinctive to my ear, but they are different enough (and about as
different as those of the other two congeners) to make me think that they
should be considered distinct, and the biogeography makes the conspecificity of
the two dubious at best. For English names, why must we just consider
plumage-based names? "Foothill Tanager" would outline the typical
distribution C. frenata well, or something like "Growling
Tanager" based on the calls one hears regularly.”
Comments
from Bonaccorso:
“NO. The data are fairly scant. Plumage differences
are within those found among conspecifics. There are not enough vocal data to
decide objectively, and molecular data are based on one sample per subspecies
for the subspecies that are represented in the phylogeny.”
Comments from Zimmer: “YES. With a nod to the concerns expressed by
Santiago and Nacho, count me in the camp that thinks the vocal differences are
different enough, even without a rigorous quantitative analysis across all
populations. Coming from the perspective
of someone who was very familiar with the Central American carmioli-group from Panama and Costa Rica, long before encountering
frenata, I was struck on my first and
all subsequent encounters with the latter, by the vocal distinctions, more so
in the calls than in the songs, which, at least in my experience, are heard
from these birds far more than are the songs.
The way that these roving gangs of tanagers leapfrog through the dark
forest understory, maintaining contact with one another through their
continuously delivered, varied, and sometimes, even explosive vocalizations,
would suggest to me that any stereotypical vocal differences between these taxa
are of magnified importance with respective to any potential or actual gene
flow, and are much more likely to be indicators of such than are the relatively
subtle plumage differences. As Van
points out, these plumage distinctions, although subtle, are on par with those
between some species-pairs of Habia (which,
in many respects, are similar to Chlorothraupis
behaviorally, and in the breadth of their vocal repertoires, as well as in
plumage and overall morphology), so the plumage similarities between carmioli (northern group) and frenata don’t really bother me. Also, as pointed out by Oscar, and reiterated
by others on the committee, the nature of the range disjunction between the
two, with two recognized (as distinct) congeners occupying the gap, requires
some mental gymnastics to square with biogeography. I’ll hold off on diving into English names in
any depth until we have a separate proposal, but I am partial to retaining
“Carmiol’s” as the name for the CA group, and I would agree with comments
expressed to the effect that using “Olive” is a non-starter.”
Comments from Pacheco: “YES. Influenced mainly by
Kevin's last comments, in which he highlights the capricious disjunction of
these two taxa.”
Additional comments from Claramunt: “I change
my vote to YES. On a second read of the proposal and inspection of images of
nominate carmioli and frenata, I realized that the plumage
differences are clear and consistent. In particular, the combination of a
yellowish face and a contrasting black bill give frenata a very distinct
look. I now think that this is yet another case in which, under the polytypic
species concepts, similar-looking taxa were lumped without any evidence of
shared ancestry or reproductive compatibility, distorting reality and producing
an artifactual biogeographic pattern.”
Comments
subsequent to 1 March 2023 update