Proposal (968) to South
American Classification Committee
Species
limits in Thamnophilus ruficapillus
Our
current SACC notes read:
"7c. Short (1975) suggested that
the Andean subspecies (marcapatae and jaczewskii) might warrant
recognition as a separate species from Thamnophilus ruficapillus, but
see Ridgely & Tudor (1994).
7f. Plumage and vocal characters
strongly suggested that Thamnophilus ruficapillus and T. torquatus
should be placed next to the T. doliatus group in linear sequences
(Ridgely and Tudor 1994), and this change in sequence was made by Zimmer &
Isler (2003), who considered them to form a superspecies. Genetic data
(Brumfield & Edwards 2007) strongly support this, with T.
ruficapillus and T. torquatus are sister species and closely related
to T. doliatus. SACC proposal passed to change linear
sequence of species."
The Rufous-capped Antshrike T. ruficapillus is polytypic, with the following 5 subspecies – and
overall distribution:
Atlantic
Forest and Parana/del Plata basin:
• T. r. ruficapillus
Vieillot, 1816 – E Paraguay and SE Brazil (S from E Minas Gerais and Espírito
Santo) S to NE Argentina (Misiones, Corrientes, Entre Ríos, NE Buenos Aires)
and Uruguay.
Andes
(from N to S)
• T. r. jaczewskii
Domaniewski, 1925 – Andes of N Peru (C Cajamarca, Amazonas S of R Marañón, NW
San Martín).
• T. r. marcapatae
Hellmayr, 1912 – E slope of Andes of S Peru (E Cuzco, Puno).
• T. r. subfasciatus
P. L. Sclater & Salvin, 1876 – E slope of Andes of NW Bolivia (La Paz, W
Cochabamba)
• T. r. cochabambae
(Chapman, 1921) – E slope of Andes of C Bolivia (E Cochabamba and SW Santa
Cruz) S to NW Argentina (Jujuy, Salta, Tucumán).
Previous treatments
Short 1975 (pages 262-263) wrote:
"?Thamnophilus ruficapillus
Rufous-capped
Antshrike
Taxonomy. Polytypic. Forms a superspecies with Andean marcapatae (disjunct populations of
latter in northern and in southeastern Peru). Thamnophilus marcapatae is darker, grayer, and less pale rufescent
than ruficapillus, and females are
very dark (rufous) below, not whitish-buff as in ruficapillus.
Ecology. Found in forest undergrowth and in canopy. Nonmigratory.
Distribution and variation. The superspecies is endemic in central
South America. Thamnophilus ruficapillus
occurs from northern Bolivia S along the Andes to Tucumán, and, disjunctly,
from Espirito Santo and eastern Paraguay S to northern Buenos Aires and
Uruguay. It occurs outside of the chaco, but is very likely to reach the
western fringes of the chaco in Salta or western Paraguay, and, in the E, it
possibly may reach the W bank of the Parana River in the Santa Fe chaco. The
eastern nominate ruficapillus is more
rufous and less gray than the western races. Of the two western forms, cochabambae occurs from central Bolivia
S, and is the one likely to occur in the western chaco fringes. It is lighter gray and less fully barred
below than is west-central Bolivian subfasciatus."
Ridgely & Tudor (1994) remarked on
the vocal similarities, highlighted the distinctiveness of marcapatae and suggested that subfasciatus
was intermediate between the northernmost Andean taxa and cochabambae, proposing that variation was clinal:


The BirdLife split:
Del Hoyo & Collar (2016), see also del Hoyo et
al. (2017), split the three north Bolivian/Peruvian taxa (subfasciatus, marcapatae and
jaczewskii) as the
Northern Rufous-capped Antshrike T.
subfasciatus with the following argument:
"Belongs to the “T. doliatus group” (which see). Hitherto
treated as conspecific with T.
ruficapillus, but differs in its denser barring on breast, this extending
to belly and onto throat, in male (3); rufous vs whitish below in female (3);
shorter tail (effect size 2.79, score 2). Three subspecies recognized."
Genetics
A
series of genetic studies, using different combinations of taxa, are consistent
among them and point to relatively deep divergences among taxa:
• Brumfield & Edwards (2007) found that a sample
of cochabambae and one of torquatus were sister (3.6% divergent in
ND2, 5.2% in Cyt b).
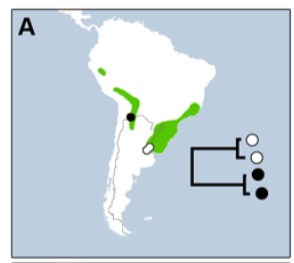
• Kerr et al. (2009) found a deep (ca. 4%)
divergence in COI between cochabambae
and ruficapillus
with two samples per taxon:
More recently, with better geographic sampling but
just one sample per taxon Harvey et al.
(2020) found T.
torquatus as sister to nominate ruficapillus,
whereas cochabambae
was sister to marcapatae
and jaczewskii
(lacking samples of subfasciatus):
![]()
Importantly, note that according to this tree T. ruficapillus would be
paraphyletic whether we follow the traditional arrangement or whether we decide
to split it along BirdLife lines. With all this in mind, one could solve the
lack of monophyly of T. ruficapillus either
by moving cochabambae as a taxon
within subfasciatus or by following a
more drastic course and performing a three-way split, separating cochabambae (monotypic) and subfasciatus (polytypic)
from a monotypic ruficapillus.
However, (a) Harvey et al. (2020) had no genetic samples of nominate subfasciatus, (b) there is
just one sample per taxon, and (c) no rigorous vocal studies have been
conducted.
Vocalizations
The vocalizations of two undisputed species, T. torquatus
and T. ruficapillus, are quite similar. However, no
formal quantitative studies have been published to date for this complex. Mark
and I have recorded cochabambae
and ruficapillus
extensively, in the hope of being able to sit down and analyze the data
properly. Although we still have to do that, our preliminary analyses (Areta
& Pearman, unpublished) indicate that there are some differences, but the
differences are far from obvious: birds in the Andes of NW Argentina (cochabambae) deliver faster paced songs
than lowland birds in NE Argentina (ruficapillus).
This is an interesting study case to apply the Isler et al. (1998) criteria,
that has been instrumental to most taxonomic changes in Thamnophilidae during
the last 25 years.
Plumage
In terms of plumage, the subfasciatus group is the most distinctive, given its extensive and
profuse ventral barring, grey flanks and back, and grey face
(https://macaulaylibrary.org/asset/204867361), whereas cochabambae has a greyish face
(https://macaulaylibrary.org/asset/205684661) that distinguishes it from the
browner-faced, yet very similar ruficapillus
(https://macaulaylibrary.org/asset/68731871). Overall, despite variation, cochabambae has less extensive ventral
barring and is paler dorsally than ruficapillus.
Ridgely & Tudor (1994) (see above) suggested that plumage varied clinally
in Andean taxa.
Brief
analysis
Given the apparent vocal conservatism in the ruficapillus/torquatus clade, we think that a full comparative study is
necessary to correctly sort out the species limits here. More genetic samples
of all taxa (especially including subfasciatus)
would also strengthen the case. Although the plumage differences (the main
features used to describe the taxa) seem well established, a more thorough
characterization of geographic variation would also be welcome, especially to
assess whether variation is clinal across the Andean taxa cochabambae-subfasciatus-marcapatae-jaczewskii. The BirdLife
treatment was based just on plumage and tail length: features that can hardly
be used as solid characters in antbird species-level taxonomy, given the number
of well-studied widespread (biological) species with striking plumage and
morphological differences that exhibit clinal variation.
Voting
options
A) Treat T. ruficapillus as a single, polytypic
species, including the 5 traditional subspecies --- The traditional treatment.
B) Split T. ruficapillus (with ssp. ruficapillus and cochabambae) from T.
subfasciatus (with ssp. subfasciatus,
marcapatae and jaczewskii)
--- The BirdLife treatment.
C) Split T. ruficapillus (monotypic), and T. subfasciatus (with ssp. subfasciatus, marcapatae, jaczewskii and cochabambae)
--- A different 2-species option that achieves monophyly for both: there would
be one Western (Andes) species and one Eastern (Atlantic Forest and Parana/del
Plata river basin) species
D) Split T. ruficapillus (monotypic), T. cochabambae (monotypic) and T. subfasciatus (with
ssp. subfasciatus, marcapatae
and jaczewskii)
--- A 3-species option that has not been suggested before in the literature to
our knowledge.
Recommendation
We recommend a YES to option A and NO to options B, C,
and D. More studies, especially including genetic samples
of nominate subfasciatus and
rigorous, large-scale comparative vocal analyses are needed to sort this
complex out adequately. Collectively, the data indicate that the current
single-species treatment seems erroneous, but we lack enough data to make fully
informed improvements to our classification.
Additional
references
del
Hoyo, J., and N. J. Collar (2016). HBW and BirdLife International Illustrated
Checklist of the Birds of the World. Volume 2. Lynx Edicions, Barcelona, Spain.
del
Hoyo, J., N. Collar, and G. M. Kirwan (2017). Northern
Rufous-capped Antshrike (Thamnophilus subfasciatus). In
Handbook of the Birds of the World Alive (J. del Hoyo, A. Elliott, J. Sargatal,
D. A. Christie, and E. de Juana, Editors). Lynx Edicions, Barcelona. Retrieved
from Handbook of the Birds of the World Alive: https://birdsoftheworld.org/hbw/species/rucant4/1.0
Kerr KCR, Lijtmaer DA,
Barreira AS, Hebert PDN, Tubaro PL. (2009) Probing evolutionary patterns in
Neotropical birds through DNA barcodes. PLoS ONE 4(2): e4379.
doi:10.1371/journal.pone.0004379
Juan I. Areta &
Mark Pearman, February 2023
Comments
from Remsen: “YES to A and NO to B, C, and D, as recommended in the
proposal. I strongly agree with the
theme of the analysis: this is a complex situation that really needs a thorough
study of voice and potential contact zones, with multiple genetic samples from
strategic locations. Making splits based
on differences in tail length and plumage in antbirds is just silly, and using genetic
distance is problematic, for conceptual and practical reasons (that I’ve
elaborated on in many previous proposals; further, the reported distances in
the taxa sampled are routine in Neotropical birds for taxa ranked as subspecies
for lack of vocal differences or evidence of free gene flow in contact zones).”
Comments
from Stiles: “YES
to A- maintain a polytypic T. ruficapillus pending more conclusive data
supporting a split.”
Comments from Del-Rio: “YES to A, and NO to B, C and D. Waiting on an updated phylogenetic
tree to clarify whether ruficapillus would be polyphyletic, for now, keep the status quo.”
Comments
from Zimmer:
“YES to Option A (continue to treat T. ruficapillus as a single,
polytypic species, including the 5 traditionally recognized subspecies.), and
NO to options B, C and D. Although the
incomplete genetic data, and striking differences in both male and female
plumage of some of the Andean taxa versus nominate ruficapillus are
certainly suggestive of more than one species-level taxon being involved, I
agree completely with Nacho and Mark that more genetic, vocal, and
morphological/plumage analyses are needed from throughout the Andean
distribution of taxa to make sense of the variation, and, to rule out clinality
in morphological and vocal characters.
This case actually reminds me a lot of the situation with Thamnophilus
caerulescens, which Mort Isler and I grappled with while working on the
Thamnophilid chapter for Volume 8 of HBW.
As is the case with ruficapillus, caerulescens is a
polytypic species (I think Peters recognized 12 ssp., but Mort and I only
recognized 8.), with a broad, and somewhat unusual distribution that includes a
chain of multiple Andean taxa, as well as Atlantic Forest representatives
ranging from NE Argentina, SE Paraguay, Uruguay, and SE Brazil (and even to NE
Brazil in Ceará and Pernambuco, so even more extensive than the distribution of
ruficapillus). As with ruficapillus-group,
the caerulescens-group encompasses a considerable amount of plumage
variation in both male and female plumages, with males of some subspecies-pairs
and females of others being exceptionally dichromatic. Also, as in the ruficapillus-group,
vocalizations of the caerulescens-group are remarkably consistent across
such a broad range, particularly given the extent of plumage variation within
the group. Our conclusion, when
researching the antbird chapter for HBW, was that, in spite of the apparent
discontinuities in diagnostic plumage characters separating many of the
described subspecies, that it would take a broadly sampled quantitative vocal
analysis to demonstrate any diagnostic vocal characters, and to eliminate the
possibility that perceived distinctions in pace of the loudsongs (which was the
only vocal difference between populations that we could discern from a
qualitative “ear test”) between some populations did not differ clinally. A couple of years later, (2005) the Islers
and Robb Brumfield published just such a study of caerulescens, albeit
one dealing only with the Andean taxa, and not with any of the Atlantic Forest
populations, and found that vocalizations did vary clinally across named
populations. In fact, as I recall, the
only diagnostic vocal character they could find was pace of loudsong (maybe
also number of notes?), and even that single character differed only between
populations at either end of the cline, with no vocal characters allowing
diagnosis of any two adjacent populations.
I thought of this as soon as I read Nacho’s comments in the Proposal
regarding the preliminary results of the vocal study that he and Mark have been
doing on ruficapillus and cochabambae – ‘some differences…far
from obvious…cochabambae delivering faster-paced songs than ruficapillus.’
“All
of this having been said, I still suspect that a similar, thoroughly sampled
genetic, vocal and plumage analysis of the various eastern (NE Brazil south to
Argentina, Paraguay and Uruguay) taxa in the caerulescens-group would
support recognition of at least one species distinct from the Andean
populations. Similarly, I would not be
surprised if such a detailed analysis across all populations of the ruficapillus-complex
would justify some splitting of taxa too, particularly since there is a vocally
similar sister species (T. torquatus) whose range basically fills the
gap between the range of nominate ruficapillus and that of the other
subspecies. But the point is, that it
will take robust analyses such as the molecular and vocal studies of caerulescens
by Brumfield (2007) and Isler et al (2005), not simplified, point-based scoring
systems of plumage or tail length, to resolve the situation in my opinion.”
Comments from LANE: A) YES.
NO to the remaining options. Until it is clear with which group
cochabambae falls, and
what (if any) characters vocal analyses can unearth, I think we should not make
changes that could require reversals once studies are performed. One issue I
think is overlooked in this proposal is the status of T. torquatus with
respect to the rest of T. ruficapillus. In the proposal, they are called
"undisputed species" but I wonder? I will
point out that T.
torquatus and
T. ruficapillus (nominate
group) have extremely limited overlap, and with birds that look like this
(https://macaulaylibrary.org/asset/112680911), (https://macaulaylibrary.org/asset/212242301), (https://macaulaylibrary.org/asset/139135831), (https://macaulaylibrary.org/asset/104308891), (https://macaulaylibrary.org/asset/203030841), (https://macaulaylibrary.org/asset/213202581), (https://www.wikiaves.com.br/2762203&t=s&s=10833&tag=FOTOALIMENTACAO) (https://www.wikiaves.com.br/922533&tm=f&t=s&s=10833&o=mp&o=mp) (https://www.wikiaves.com.br/1313378&tm=f&t=s&s=10833&o=mp&o=mp) (https://www.wikiaves.com.br/1317945&tm=f&t=s&s=10833&o=mp&o=mp) (https://www.wikiaves.com.br/3185750&tm=f&t=s&s=10833&o=mp&o=mp) (https://www.wikiaves.com.br/1761206&tm=f&t=s&s=10833&o=mp&o=mp) (https://www.wikiaves.com.br/2786202&tm=f&t=s&s=10833&o=mp&o=mp) (https://www.wikiaves.com.br/2748024&tm=f&t=s&s=10833&o=mp&o=mp) (https://www.wikiaves.com.br/1744876&tm=f&t=s&s=10833&o=mp&o=mp) (https://www.wikiaves.com.br/2642126&tm=f&t=s&s=10833&o=mp&o=mp) (https://www.wikiaves.com.br/1644632&tm=f&t=s&s=10834&o=mp&o=mp)
at sites where both species
reportedly occur makes me wonder just how “undisputed” the species status of
T. torquatus really is?
Other than the black cap of the male, I don’t see or hear a whole lot that
distinguish the two… and is that black cap as important a feature in the birds’
eyes as it seems to be for us? My point is: I am not entirely convinced that we
should be leaving T.
torquatus out of this complex simply
because it looks so distinctive to human eyes. It may be best inserted into
T. ruficapillus,
and then the paraphyly of the latter is no longer! According to
Wagner Nogueira, it seems
that the present-day contact of the two taxa is thanks to recent anthropogenic
habitat alteration, so a broad region of contact and overlap is not to be
expected, and the above evidence of potential interbreeding could be a
precursor of a larger future swarm.”
Comments from Rafael Lima: “I
agree with Daniel that it may be best to lump T. torquatus with the
T. ruficapillus complex, at least
provisionally while thorough genetic and acoustic trait analyses are not
available. Although this possibility has not been considered in the
original proposal, it would equally eliminate paraphyly.
“I have recently cursorily
examined dozens of specimens of almost all taxa of the complex at the
AMNH, LSUMNS, and MZUSP, and my impression is that if two biological species
were to be recognized in this complex for the time being, based on morphology
alone, these species should be the eastern taxa (ruficapillus + torquatus)
comprising one species and the Andean taxa (subfasciatus + marcapatae
+ jaczewskii + cochabambae) comprising the other species.
Subspecies cochabambae is difficult to place in one of the two
morphologically-delimited species, but I think this arrangement makes much more
sense than treating the Andean taxa plus ruficapillus as one species and
torquatus as another. This arrangement would also result in two monophyletic
species taxa. Nevertheless, I think that based on current evidence (including
the many photos on WikiAves suggesting that torquatus and ruficapillus
hybridize freely where they come into contact), the best option is to treat all
of them as a single biological species until a thorough study of geographic
variation is available.”
Comments from Robbins: “YES to A, no to B, C, D for
reasons outlined in the proposal and based on comments by other committee
members.”
Comments from Claramunt: “Option A. The situation is
clearly complex and requires a detailed analysis of the evidence. Nothing new
has been published. NO to B,C,D.”
Comments from Bonaccorso: “A, YES. No to B, C, D. The
problem with the last three proposals is not only the lack of vocal data (as
the proposal indicates), but there is also a lack of good geographic and
genetic sampling across the ranges of all taxa involved in the potential alternatives.”