Proposal (970) to South
American Classification Committee
Note from Remsen: This proposal is being evaluated by NACC concurrently
Revise
generic limits among Rhodothraupis, Periporphyrus, and Caryothraustes,
and adopt a new linear sequence for these taxa
Background:
Rhodothraupis celaeno (Deppe, 1830) is a
dichromatic understory cardinalid of the lowlands of northeastern Mexico, the
males being largely dark red with a solid black head and dark back, whereas the
females have the red replaced by yellowish olive (Brewer 2020b). This overall
plumage pattern is shared by Periporphyrus erythromelas ("Gmelin,
JF", 1789) of northeastern South America and the southeastern Amazon
Basin, although that species is brighter red (males) or yellow (females),
especially on the back, and has a larger bill (Brewer 2020a, eBird records).
After being placed in various ‘catchall’ genera (e.g., Loxia, Fringilla)
in the 19th century, Rhodothraupis celaeno bounced around
between the monotypic genus Rhodothraupis Ridgway 1898, Periporphyrus
Reichenbach 1850, Caryothraustes Reichenbach 1850, and Pitylus
Cuvier 1829 (Ridgway 1901, Hellmayr 1938), whereas Periporphyrus
erythromelas moved between its current genus and Pitylus Cuvier 1829
(Hellmayr 1938). Molecular data have now shown Pitylus to be part of Saltator
in the Thraupidae, but the remaining three genera are closely related and part
of the Cardinalidae (Barker et al. 2015). Parkerthraustes humeralis
(Lawrence 1867) was previously considered a Caryothraustes until
molecular data showed it belonged in the Thraupidae (Demastes and Remsen 1994).
In the current treatment of NACC/SACC, Caryothraustes contains two
species: poliogaster of Mexico and Central America and canadensis
of northeastern South America, southeastern Brazil, and eastern Panama
(Clements et al. 2022). Both species of Caryothraustes are
monochromatic, largely arboreal lowland species. Both Rhodothraupis celaeno
and Periporphyrus erythromelas are mostly understory / midstory species,
and at least Periporphyrus will join mixed flocks (Brewer 2020a).
All four species in this group give
leisurely whistled songs in short but widely separated strophes (i.e., typical
cardinalid songs), although the pattern differs between species. The two Caryothraustes
also give a variety of “loud and arresting” calls when flocking, described as a
“zzzrt”, “tree-dreek”, or “chew-chew-chew” (Gulson 2020). Rhodothraupis and
Periporphyrus both give more subtle calls; a “high, clear, penetrating
slurred ‘sseeuu’” in Rhodothraupis and a ”high-pitched, sharp ‘spink’”
in Periporphyrus (Brewer 2020a, 2020b).
The last major generic revision of this
group was that of Ridgway (1901), resulting in the treatment followed by most
subsequent authors, with Rhodothraupis and Periporphyrus each
being monotypic, and Caryothraustes with two species (e.g. Dickinson and
Christidis 2014, Gill et al. 2020, Clements et al. 2022, Chesser et al. 2023).
Paynter (1970) lumped Caryothraustes poliogaster with canadensis,
but that treatment was not followed by subsequent authors. SACC recently
considered a proposal to split Caryothraustes canadensis, which
did not pass. In addition to whether the species were dichromatic or
monochromatic, Ridgway (1901) used the following structural characters to
delimit genera: Periporphyrus with a culmen longer than the tarsus,
concave mandibular tomium, and a “broad truncated prominence” at the base of
the tomium (i.e. a “toothed” tomium), Rhodothraupis with a relatively
longer tail and narrower bill, and Caryothraustes with a relatively
shorter tail and broader bill. We now know that bill shape is extremely labile
in the Cardinalidae, with a particularly drastic example being “Guiraca”
[=Passerina] caerulea.
del Hoyo et al. (2016) transferred both Rhodothraupis
and Periporphyrus to Caryothraustes but provided no rationale
for this action. Both Periporphyrus and Caryothraustes were
described in the same volume by Reichenbach (Reichenbach 1850), but I am unable
to locate a copy of this volume to review the genus descriptions. Hellmayr
(1938) lists the two genera as being described on sequential plates 77 (Periporphyrus)
and 78 (Caryothraustes). My interpretation is that these genera were
therefore simultaneously published, and that in transferring Periporphyrus
to Caryothraustes, del Hoyo et al. (2016) should be considered the First
Revisers when they selected Caryothraustes as having priority.
New Information:
Barker et al. (2015) used a supertree
approach to estimate a phylogeny for the 9-primaried oscines which resulted in
many genus and family level rearrangements that have since been adopted by
NACC, SACC, and other authorities. This tree was based primarily on the
mitochondrial genes Cyt-B and ND2, augmented by four nuclear genes for
representatives of most genera. Below I have reproduced a portion of the tree
showing most of the Cardinalidae, including the species relevant to this
proposal (Figure 1). Of note is that Rhodothraupis, Periporphyrus,
and Caryothraustes form a clade sister to Cardinalis. Rhodothraupis
and Periporphyrus are sister taxa, separated by about 5 Ma (right-most
dashed line in Figure 1). A more recent phylogenetic analysis of Caryothraustes
(Tonetti et al. 2017; with Rhodothraupis and Periporphyrus as
outgroups) using the mitochondrial locus ND2 found similar branch lengths and
topology as Barker et al. (2015).

Figure 1. A portion of
the Cardinalidae phylogeny from Barker et al. (2015). The two vertical dashed
lines correspond to 10 Ma (left-hand line) and 5 Ma (right-hand line). Rhodothraupis
celaeno is indicated with a red arrow.
A more recent study on sister
relationships of multiple complexes of Amazonian and Atlantic Forest taxa
(Bocalini et al. 2021) included all subspecies of Caryothraustes and
both Rhodothraupis and Periporphyrus as outgroups. They estimated
a coalescent-based species tree from 3,826 UCE SNPs, which I have reproduced
below (Figure 2). As an aside, Bocalini et al. (2021) found that Caryothraustes
canadensis simulans of eastern Panama was sister to C. poliogaster,
rather than to the remainder of C. canadensis, so a species-level
taxonomic change should be considered for simulans, either considering
it a separate species or transferring it to C. poliogaster.
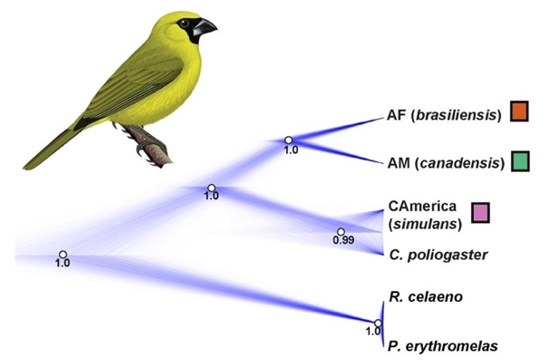
Figure 2. The phylogeny
of Caryothraustes and two outgroups, estimated in SNAPP. From Bocalini
et al. (2021).
The topology of the phylogenies in
Barker et al. (2015) and Bocalini et al. (2021) are concordant, but the branch
lengths are extremely different. Most notably, the UCE tree in Bocalini et al.
(2021) found extremely low divergence between Rhodothraupis and Periporphyrus
(far less than the divergence within Caryothraustes),
although I note that this is a coalescent-based analysis, which in my
experience often recovers lower divergence estimates than maximum likelihood
methods like those used in Barker et al. (2015). The Barker et al. (2015) study
was based on many fewer loci, which may also explain the different branch
lengths between the two studies.
A series of specimens of the relevant
species in this group are shown on below, courtesy of Terry Chesser. Within
each species, males are on the left and females on the right, and the species
from left to right are: Rhodothraupis celaeno, Periporphyrus
erythromelas, Caryothraustes poliogaster, and Caryothraustes canadensis.



Effect on AOS-CLC area:
Merging Rhodothraupis into Periporphyrus
would result in a name change from Rhodothraupis celaeno to Periporphyrus
celaeno. Merging all species into Caryothraustes (following del Hoyo
et al. 2016) would result in name changes for Rhodothraupis celaeno and Periporphyrus
erythromelas in the following linear sequence: Caryothraustes celaeno,
C. erythromelas, C. poliogaster, C. canadensis.
Recommendation:
The two major clades in this group have
the same number of species (2), and Rhodothraupis celaeno is the
northern-most member of the group, so regardless of any genus-level transfers celaeno
should go first in the linear sequence. I recommend adopting a new linear
sequence (see below), which differs only slightly from the current NACC
treatment.
The ~5 Ma divergence between Rhodothraupis
and Periporphyrus in Barker et al. (2015) is less than that shown by
most related cardinalid genera, and the very low divergence between the
two species found by Bocalini et al. (2021) suggests that these two species are
very closely related. The bill size / shape differences are best not considered
genus-level characters in the Cardinalidae, and I find the wing and tail length
differences to not be drastically different. Combined with the broadly similar
red-and-black (male) and green-and-black (female) plumages of Rhodothraupis and
Periporphyrus, I think Rhodothraupis celaeno is best transferred
to Periporphyrus.
Based solely on relative branch lengths
in Barker et al. (2015), the divergence between Caryothraustes and Rhodothraupis
+ Periporphyrus is roughly comparable to some other genus-level
divergences in the Cardinalidae, such as those among Amaurospiza,
Cyanoloxia, and Cyanocompsa. These similar genus-level clade ages,
combined with the differing plumage dimorphism (monochromatic in Caryothraustes
vs. dichromatic in Rhodothraupis and Periporphyrus), and a
more canopy-dwelling habit and differing calls of Caryothraustes, are
sufficient in my view to keep Caryothraustes and Periporphyrus as
separate genera. Although I minimized the importance of the bill shape
differences in advocating for the merger of Rhodothraupis and Periporphyrus,
the two Caryothraustes do have a notably wide bill. Caryothraustes
do also look superficially like females of Rhodothraupis and Periporphyrus,
albeit with restricted black on the head. That said, I don’t think that a
merger of Caryothraustes and Periporphyrus is necessary, although
it would maintain a monophyletic grouping.
Please vote on the following:
A. Adopt the following linear sequence: celaeno (extralimital),
erythromelas, poliogaster (extralimital), canadensis.
B. Transfer Rhodothraupis celaeno (extralimital) to
Periporphyrus (advisory only)
C. Transfer Rhodothraupis celaeno and Periporphyrus
erythromelas to Caryothraustes (only if B passes)
I recommend a YES on A and B, and
a NO on C.
Literature Cited:
Barker, F.
K., Burns, K. J., Klicka, J., Lanyon, S. M., and Lovette, I. J. 2015. New
insights into New World biogeography: An integrated view from the phylogeny of
blackbirds, cardinals, sparrows, tanagers, warblers, and allies. The Auk,
132(2), 333–348. https://doi.org/10.1642/AUK-14-110.1
Bocalini,
F., Bolívar-Leguizamón, S. D., Silveira, L. F., and Bravo, G. A. 2021.
Comparative phylogeographic and demographic analyses reveal a congruent pattern
of sister relationships between bird populations of the northern and
south-central Atlantic Forest. Molecular Phylogenetics and Evolution, 154,
106973. https://doi.org/10.1016/j.ympev.2020.106973
Brewer, D.
2020a. Red-and-black Grosbeak (Periporphyrus erythromelas), version 1.0.
In Birds of the World (J. del Hoyo, A. Elliott, J. Sargatal, D. A. Christie,
and E. de Juana, Editors). Cornell Lab of Ornithology, Ithaca, NY, USA. https://doi.org/10.2173/bow.rabgro1.01
Brewer, D.
2020b. Crimson-collared Grosbeak (Rhodothraupis celaeno), version 1.0.
In Birds of the World (J. del Hoyo, A. Elliott, J. Sargatal, D. A. Christie,
and E. de Juana, Editors). Cornell Lab of Ornithology, Ithaca, NY, USA. https://doi.org/10.2173/bow.crcgro.01
Chesser,
R. T., S. M. Billerman, K. J. Burns, C. Cicero, J. L. Dunn, A. W. Kratter, I.
J. Lovette, N. A. Mason, P. C. Rasmussen, J. V. Remsen, Jr., D. F. Stotz, and
K. Winker. 2023. Check-list of North American Birds (online). American
Ornithological Society.
Clements,
J. F., T. S. Schulenberg, M. J. Iliff, T. A. Fredericks, J. A. Gerbracht, D.
Lepage, S. M. Billerman, B. L. Sullivan, and C. L. Wood. 2022. The
eBird/Clements checklist of Birds of the World: v2022. Downloaded from https://www.birds.cornell.edu/clementschecklist/download/
Demastes,
J. W., and J. V. Remsen, Jr. 1994. The genus Caryothraustes
(Cardinalinae) is not monophyletic. Wilson Bulletin 106(4): 738–743.
Dickinson,
E. C., and L. Christidis (Editors) (2014). The Howard and Moore Complete
Checklist of the Birds of the World. 4th edition. Volume Two.
Passerines. Aves Press Ltd., Eastbourne. UK.
Gill, F.,
D. Donsker, and P. C. Rasmussen (Editors) (2020). IOC World Bird List (v 10.2).
DOI 10.14344/IOC.ML.10.2. http://www.worldbirdnames.org/
Gulson, E.
R. 2020. Black-faced Grosbeak (Caryothraustes poliogaster), version 1.0.
In Birds of the World (T. S. Schulenberg, Editor). Cornell Lab of Ornithology,
Ithaca, NY, USA. https://doi.org/10.2173/bow.blfgro1.01
Hellmayr,
C. E. 1938. Catalogue of birds of the Americas, part XI. Field Museum of
Natural History Zoological Series Vol. XIII. Chicago, USA.
del Hoyo,
J., Collar, N.J., Christie, D.A., Elliott, A., Fishpool, L.D.C., Boesman, P.
and Kirwan, G.M. 2016. HBW and BirdLife International Illustrated Checklist of
the Birds of the World. Volume 2: Passerines. Lynx Edicions and BirdLife
International, Barcelona, Spain and Cambridge, UK.
Paynter,
R. A., JR. 1970. Subfamily Cardinalinae. Pp. 216-245 in Check-list of birds of
the world (R. A. Paynter, Jr., ed.). Museum of Comparative Zoology, Cambridge,
Massachusetts
Reichenbach,
H. G. L. 1850. Avium systema naturale. Das natürliche System der Vögel.
Expedition der vollständigsten naturgeschichte. Dresden and Leipzig.
Ridgway,
R. 1901. The birds of North and Middle America. Part I. Bulletin of the United
States National Museum. No. 50.
Tonetti,
V. R., F. Bocalini, L. F. Silveira, and G. Del-Rio. 2017. Taxonomy and
molecular systematics of the Yellow-green Grosbeak Caryothraustes canadensis
(Passeriformes: Cardinalidae). Revista Brasileira de Ornitologia 25: 176–189.
Oscar Johnson,
February 2023
Vote tabulation:
https://www.museum.lsu.edu/~Remsen/SACCPropChart864+.htm
Comments
from Robbins: “YES. I support the new linear sequence proposed by Oscar and
also vote Yes for the rationale he presents in placing Rhodothraupis celaeno
in Periporphyrus. I would support
either maintaining Periporphyrus or placing it within Caryothraustes.”
Comments
from Stiles: “YES
to all recommendations. A- the new sequence based on geography fits with the
closer affinities between Periporphyrus and Rhodothraupis than with Caryothraustes; B- genetic,
plumage and ecological similarities lend support to merging Rhodothraupis
into Periporphyrus, and C- vocal, genetic and ecological differences
support maintaining separate Periporphyrus from Caryothraustes.”
Comments from Areta:
“A-YES to the proposed linear sequence.
“B-NO. I don´t see a pressing need to move
Rhodothraupis into
Periporphyrus. My main arguments have to deal with
side-effects that I think should be considered with a broader comparative
framework in mind. This seems a borderline case to me in which any option can
be defended, but merging them should (from my perspective)
lead to a cascade effect also
requiring decisions on what to do with other genera, given the lack of
monophyly of Habia, and the
deep divergences in Piranga. I think
that it would be more appropriate to deal with the
Rhodothraupis/Periporphyrus
case after a comparative study is published dealing with generic
limits in this clade. I find it difficult to decide what to do with the long
branch length of Rhodothraupis/Periporphyrus,
without further context on generic limits in the group. For
example, H. rubica
seems to have diverged from
Chlorothraupis at about
the same time as Rhodothraupis
and Periporphyrus, and two
even more deeply diverged clades have been uncovered in Piranga. I feel
that we need a better comparative internal yardstick in order to decide. Otherwise,
this merger might (or might not) need to be undone soon. I am not strongly
opposed to merging Rhodothraupis
into Periporphyrus, it is
just that I do not know how other pieces in the puzzle will fit and I prefer to
have the advantage of a broader comparative look before deciding to change this
well-entrenched taxonomy.
“C-NO. Pretty much for the same reasons that I voted NO to B: one
could choose to accommodate the generic limits in different ways depending on
different criteria, but I do not think it is advisable to mess around with
Rhodothraupis/Periporphyrus/Caryothraustes
without a clear perspective on what to do with other close
relatives. For example, one could follow the merger of
Rhodothraupis and
Periporphyrus into
Caryothraustes and then
also decide to merge Habia with
Chlorothraupis and
recognizing a deeply diverged Piranga. I would
possibly vote against all these three if the moment arrives, but this would be
another way of dealing with generic limits in this clade. An integrative
perspective on genetic and phenotypic variation in this clade should give us
more elements to make a balanced decision towards establishing generic limits.”
Comments from Lane: “A) YES.
B) NO, I don’t see a need to do this, given the long branches involved and the
distinctions between the two species. C) NO, as per B, this seems an
unnecessary move given the distinctiveness of the taxa involved.”
Comments from Claramunt: “I broadly concur with
Oscar’s assessment and recommendation:
“A – YES
“B – YES. erythromelas
and celaeno are two sister species with similar morphologies, plumage,
and ecology. Treating them in the same genus makes total sense. The genus
category is most useful when it groups multiple species indicating
relationships and monophyletic groups. Monotypic genera fail at that. I know
that monotypic genera are sometimes unavoidable, but this is a case in which
they are avoidable, and a single genus with two species in it makes a lot of
sense.
“C – NO. If
it were only for their plumage, I would merge all fours species into a single
genus, but I think it makes more sense to maintain the big and red erythromelas
and celaeno separated from the smaller, yellow, and canopy-dwelling Caryothraustes.”
Comments from Remsen:
“A. YES. Required book-keeping.
“B. YES.
The genetic differences are minor, and the differences in plumage insufficient
on their own to assign them to different genera.
“C. NO. The estimated
divergence time is early Miocene, well within the range of lineage ages of most
groups considered to be in separate genera, so I see no objective reason to
upset the status quo
Comments
from Zimmer:
“A) YES on adopting the
proposed linear sequence of celaeno, erythromelas (NOTE that it
is celaeno that is extralimital, not erythromelas as stated in
the Proposal), poliogaster, canadensis.
“B) YES on transferring
Rhodothraupis celaeno to Periporphyrus, as supported by multiple
data sets.
“C) NO, on folding Rhodothraupis
celaeno and Periporphyrus erythromelas into Caryothraustes. In addition to all of the differences between
these two species-pairs cited by Oscar, I would add that both Caryothraustes
troop through the forest in noisy groups of up to 20 (or more) individuals –
very unlike celaeno or erythromelas, which, at least in my
experience, tend to be encountered as rather retiring, inconspicuous pairs or
individuals.”
Comments
from Bonaccorso:
“YES on A. The phylogenies are concordant. I wouldn't
worry too much about the differences in branch lengths since we are basically
comparing a mitochondrial-DNA-driven tree with a coalescent-based tree. Based
on plumage dichromatism, and overall size and bill size, transferring Rhodothraupis
celaeno to Periporphyrus seems
reasonable, so YES to B. No to C.”
Comments from Gustavo Bravo (who has Del-Rio vote): “Given
the evidence from various datasets, I support keeping the two larger-sized and
sexually dichromatic (red/yellow) species in one genus and the smaller and
sexually monochromatic in another. Species limits within Caryothraustes
will have to be revisited in the future. Thus, my vote goes as follows:
A – YES.
B – YES.
C– NO.”
Comments
from Stiles:
“YES to A + B, NO on C. I especially dislike lumping all of these in Caryothraustes,
which differs in plumage, vocalizations and habits from Periporphyrus.