Proposal (986) to South
American Classification Committee
Note from Remsen: This proposal has also been
submitted to the North American Classification Committee
Split Anas crecca (Green-winged Teal/Common or
Eurasian Teal) into two species: A.
crecca (Common Teal or Eurasian Teal) and A. carolinensis (Green-winged Teal)
Effect on NACC (and
SACC):
This would change our treatment of the Green-winged
Teal (Anas crecca), a Northern
Hemisphere species, by splitting it into a largely New World species (Anas carolinensis; Green-winged Teal)
and a largely Old World species (Anas
crecca; Common Teal or Eurasian Teal). A.
carolinensis would be monotypic; A.
crecca would include A. c. crecca
and the sometimes-recognized Aleutian population A. c. nimia.
Background
and new evidence:
We are revisiting this species limits issue in association with the effort
to harmonize world lists,
and treatment of A. crecca is a point
of disagreement. As it turns out, this now fairly well-known group represents
an interesting tale of species concepts, methodological limitations, and modes
of speciation, each of which has influenced decisions on species limits.
AOU/AOS has considered A. c. crecca and A. c. carolinensis as a single biological species since 1973 (AOU
1973). The supporting citations, however, simply treated the forms as a single
species and did not provide direct evidence (Delacour & Mayr 1945,
Gabrielson & Lincoln 1959, Johnsgard 1965). This treatment followed Peters
(1931), an early example of the application of the biological species concept
to forms previously treated as full species. Later, justification for this
treatment was given as “intergradation between the two groups occurs in the
Aleutians.” (AOU 1983:74).
Sangster et al. (2001)
reviewed phenotypic and genetic evidence and recommended splitting crecca and carolinensis into two biological species, which was later done
(Sangster et al. 2002). They gave strong weight to the lack of a mitochondrial
DNA sister relationship between crecca
and carolinensis, and they gave
little weight to the existence of hybrids—including considering the breeding
ranges to be allopatric, apparently missing Hanna (1920), Gabrielson &
Lincoln (1959), and Murie (1959). The mitochondrial
relationship shown by Johnson & Sorenson (1999) seems to have been
particularly compelling, with the South American A. ‘flavirostris’ being sister to carolinensis, and crecca
being sister to these two: i.e., (crecca(carolinensis,flavirostris)); more on this
below. This was at about the time that systematists became aware of the
unreliability of mtDNA to accurately track organismal lineage divergence at
these shallower levels (e.g., Funk & Omland 2003), although over-reliance
on that single-locus approach, termed ‘mtDNA myopia’ by Remsen (2010, 2015),
still appears 20 years later. Quite a bit of research has been done on these
teal since Sangster et al. (2001, 2002) determined that they should be split
into two biological species.
Hybrids between crecca and carolinensis have long been known, and have been described from
both eastern and western North America and western Europe (e.g., Cruickshank
1936, Poole 1940, Mayr & Short 1970, Vinicombe
1994, Gibson & Byrd 2007). But these are ducks, after all, in which
hybridization is well known between species, so it is understandable that
hybrids might be given less weight without more detailed knowledge of the
frequency of their occurrence. But, as it happens, they are rather frequent.

(Gibson & Withrow
2015: fig. 3)
IOC has considered the
two taxa to be separate species since version 1.0 (Gill & Wright 2006).
Interestingly, HBW-BirdLife considered the two to be a single species both in
the first HBW volume (del Hoyo et al. 1992) and again after application of the
Tobias et al. (2010) species limits criteria in del Hoyo & Collar (2014).
Application of these criteria brought new evidence to the subject, so it is
worth relating that and the interpretation here:
“Race carolinensis sometimes
considered a full species, and situation finely balanced. The male differs from
nominate male in its vertical white breast-side line (2), lack of white
horizontal scapular stripe (2), and lack of narrow buff supercilium (above
broad green “eyestripe”) (1); various other very
minor differences cannot be scored (plumage characters capped at three),
differences in measurements do not exist, behavioural
differences are matters of frequency rather than type1616, and
genetic evidence, while suggesting paraphyly involving A. flavirostris868, indicates that hybridization1861
is relatively widespread in Beringia1368 (possible score for broad
hybrid zone 1) leaving carolinensis
extremely close to species status.” [Numbers refer to del Hoyo & Collar
(2014) references.]
Given the evidence then
available, that was a good outcome. But for some reason it was decided to try
again with the Tobias et al. (2010) criteria, and a different outcome was
achieved. From HBW-BirdLife (2020):
“Common Teal A.
crecca (del Hoyo & Collar 2014) has been split into Common Teal A. crecca and Green-winged Teal Anas carolinensis (Handbook of the Birds
of the World and BirdLife International 2020). This change follows a revision
to the scoring of the males' vertical white breast-side line, due to its role
as a signal in display. As such the revised scoring is as follows: male A. carolinensis differs from A. crecca male in its vertical white
breast-side line, replicated on rear flank (3), lack of white horizontal
scapular stripe (2), and lack of narrow buff supercilium (above broad green “eyestripe”) (1); various other very minor differences
cannot be scored (plumage characters capped at three), differences in
measurements do not exist, behavioural differences
are matters of frequency rather than type, and genetic evidence, while
suggesting paraphyly involving A.
flavirostris, indicates that hybridization is relatively widespread in
Beringia (allow 1 for broad hybrid zone), indicating that carolinensis does warrant species status.”
Thus, through a change
in the scoring value of one plumage character (from 2 to 3) and rigid adherence
to a methodological rubric, the two became full species. Diminishing the
importance of hybridization is a well-recognized weakness of the Tobias et al.
(2010) criteria (e.g., Winker 2010, Remsen 2015), but inadequate consideration
of by now considerable evidence of levels of hybridization is surprising. I am
not sure how widespread hybridization can be considered to warrant species
status, but given the current state of knowledge this case seems to be one in
which methodological constraints or limitations and corresponding decisions
triggered a faulty decision under the BSC. One could argue that these
constraints now include two facets: a strong preference for a cladistic view of
mtDNA gene trees dictating species limits, and a rigid adherence to character
scoring and related accounting preventing a full accounting of highly relevant
data on hybridization rates. (As a reminder, the Tobias et al. [2010] criteria
do not include hybrid zones in their divergence threshold calculations, but
they do receive a score. Their “broad hybrid zone” is scored the lowest; i.e.,
is least indicative of species status; see their table 1. Hybridization
frequency, which is the most important attribute of the phenomenon, is not
considered.)
Hybridization,
determined through male plumage, appears to be routine in the eastern Aleutians
where the ranges of the two taxa come together, and intergrades also appear on
other Bering Sea islands (Gibson & Byrd 2007:35; Gibson & Withrow 2015:
fig. 3; DeCicco 2008; Lehman 2019). Co-occurrence of both taxa on the Chukchi
and Seward peninsulas suggests hybridization might occur there as well (Kessel
1989, Konyukhov 2015). Palmer (1976) reviewed the
occurrences of other intergrades in Colorado, California, and Japan. While the
published literature provides ample evidence of hybridization, estimates of the
frequency of individuals showing hybrid characteristics has become clearer. Reeber (2015) considered hybrids to not be very common,
because they are detected with a frequency similar to the small numbers of
males found on the wrong continent (citing Sibley 2011). Actually, that’s a
rather high ratio of hybrids to the rarer parental form (i.e., ~1:1). The
expected value if reproductive isolation has essentially been achieved should
be very low. Having it be approximately equal to the number of the rarer
parental form seems quite high, because in a stable population it is indicative
of a rate of hybridization roughly equivalent to the number of opportunities for
it. This, and the occurrence of hybrids where the two taxa are in contact,
suggests that any isolating mechanisms are providing fairly ineffective
barriers.
The seemingly high
frequency of hybrids became jaw-droppingly obvious with the advent of eBird,
where Green-winged Teal (Eurasian x American), Anas crecca crecca x carolinensis has its
own page, with, at this time, 546 entries (with an abundance of photographs),
showing concentrations in western and eastern North America and western Europe
(see figure below; https://ebird.org/species/gnwtea1). Based on phenotype,
it seems obvious that these taxa have not achieved essential reproductive
isolation.
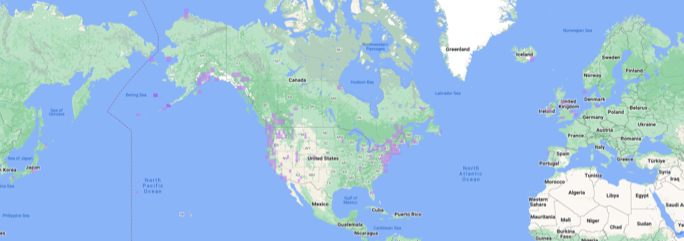
Green-winged Teal
(Eurasian x American) Anas crecca crecca x carolinensis,
global distribution of 546 records (https://ebird.org/species/gnwtea1).
Genetic information on
relationships among A. c. crecca
(including nimia),
A. c. carolinensis, and A. ‘flavirostris’ has been accumulating
for almost three decades. In terms of species limits, it is simply icing on the
cake of phenotypic evidence for substantial hybridization. Beyond that,
however, genetic and genomic data provide incomparable insights into the surprising
variety of evolutionary processes and modes of speciation occurring in this
small group.
Using mtDNA RFLPs, Zink
et al. (1995) found relatively deep divergence between Russian and U.S.
haplotypes, but also evidence that there was gene flow. Johnson & Sorenson
(1999) found a deep mtDNA divergence between crecca and carolinensis,
and also that the latter was sister not to crecca
but rather to the South American A.
‘flavirostris’, as noted above. At that time, an overcommitment to
cladistic methodology in our discipline, in which paraphyletic mtDNA
relationships for species are not allowed, often had a heavy influence (as in
this case) on species delimitation. Haffer (1992) showed how this conceptual
constraint could produce the wrong answer when considering biological species.
Since then we have come to recognize that gene trees often disagree with
species trees, and this is a case where it is good to revisit the undue weight
that this was given in historic decisions to split these taxa (e.g., Sangster
et al. 2001).
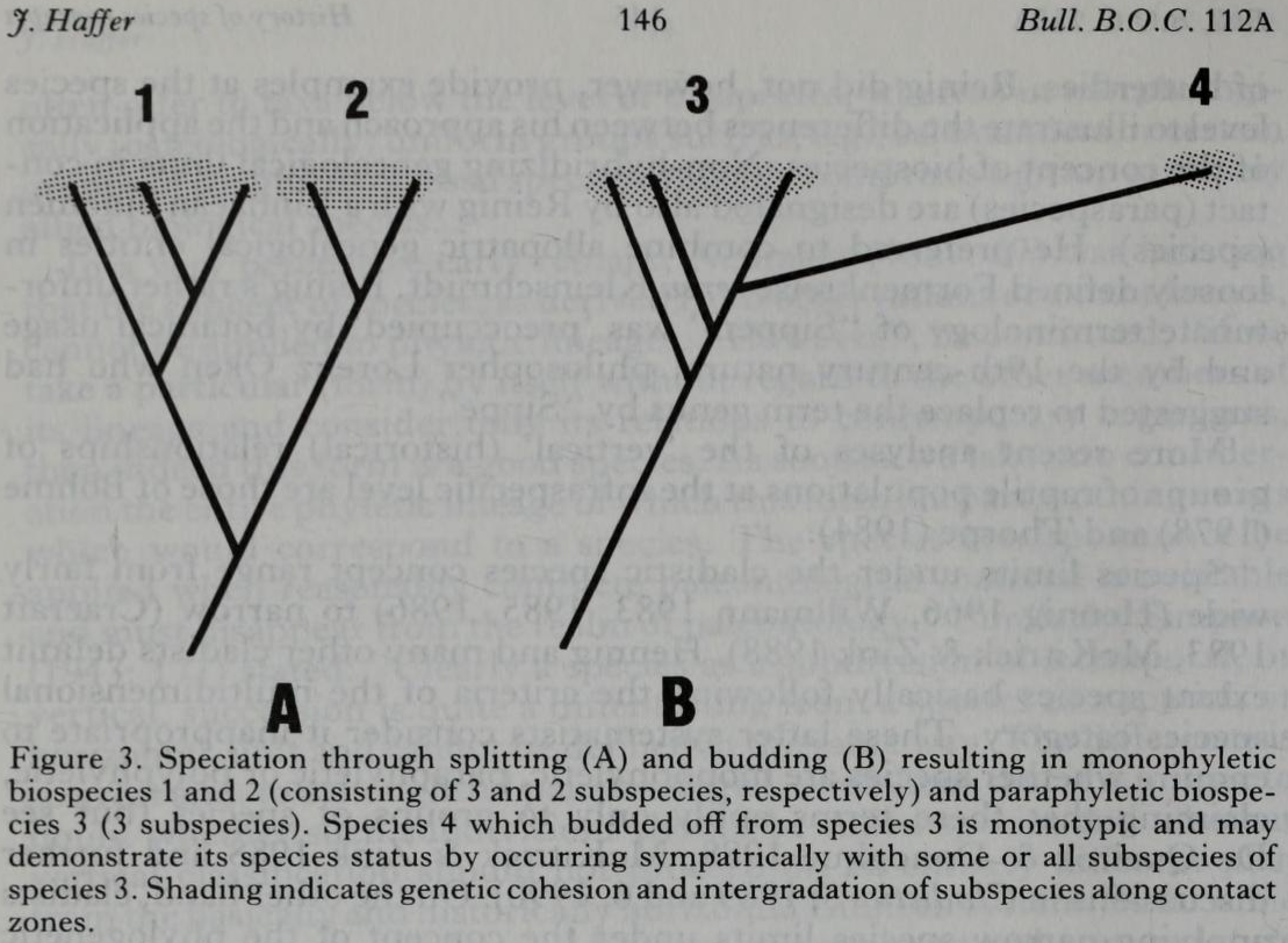
Haffer (1992: fig. 3).
Humphries & Winker
(2011) confirmed substantial divergence in mtDNA sequence (ND2) between crecca and carolinensis, but they found no significant difference between them
using 420 AFLP loci (presumed to be predominantly nuclear DNA). This indicates
less divergence in the nuclear genome.
Peters et al. (2012:
fig. 1).
Peters et al. (2012)
considered these two continental populations in the context of the classic
‘dumbbell’ model of allopatric speciation, in which, at its extreme,
populations are separated by a barrier that precludes gene flow (Mayr 1940,
1942; White 1978; Haffer 2007). If that barrier is insufficient and gene flow
persists, divergence might be retarded, and speciation could fail to go to
completion. In such a case, i.e., when the ‘handle’ connecting the two
populations in the dumbbell model is not broken (gene flow persists), then
parapatric models apply (speciation with gene flow in a nonsympatric
distribution; Gavrilets 2004). Using sequence data
from mtDNA (control region) and eight nuclear introns, Peters et al. (2012)
found that crecca and carolinensis did indeed fit a parapatric
model of speciation, and that while they appear to have been diverging for ~2.6
My in mtDNA, gene flow has been sufficiently high to prevent completion of the
speciation process under the BSC.
The natural history of
these birds is of great interest here. They form breeding pairs on the
wintering grounds (unlike most migratory birds), and males follow their mates
back to her breeding grounds. This produces female-biased philopatry or
male-biased dispersal. And, because mtDNA is maternally inherited,
intercontinental phylogeographic mtDNA structure is very high and mtDNA gene
flow is relatively low (~1/generation). In contrast, males disperse between
continents at a much higher rate, and nuclear gene flow is moderate (~1-20
individuals/generation, with an asymmetric bias (appearing in this dataset)
from crecca into carolinensis (Peters et al. 2012). Winker (2021) considered the
situation between crecca and carolinensis to be an example of an
evolutionary tryst, with divergence stalled for long periods short of
speciation: almost-separate entities, but unbreakably joined by gene flow.
The most comprehensive study of these taxa thus far used 1,393
ultraconserved element (UCE) nuclear loci and complete mitogenomes to examine
relationships in the whole complex (including the South American A. flavirostris/andium) and gene flow among the North American members (Spaulding
et al. 2023). Although this study used small sample sizes, coalescent theory
and an empirical study of sample size effects showed that key demographic
parameters (in this case levels of gene flow, Nem), are
robustly estimated using these methods (Felsenstein 2005, McLaughlin &
Winker 2020).
A note on the South
American A. ‘flavirostris’ is
warranted: SACC considers it to be two species, Anas flavirostris and A.
andium, following passage of a proposal in 2008 in which differences in
bill color largely drove the decision; see Remsen et al. (2023) and associated
comments and links. The genetic situation there has yet to be fully resolved,
but the taxa appear to be genetically differentiated (e.g., Spaulding et al.
2023). For the purposes of this proposal, it does not matter whether this South
American lineage is treated as one species or two.
Spaulding et al. (2023) found gene flow rates (Nem) of
10-11 individuals per generation between crecca
and carolinensis (with evidence for
cyclic contact likely related to glacial cycles), and 1-26 individuals per
generation between nimia
and carolinensis (the latter value
reflects gene flow from carolinensis
into nimia
and is consistent with phenotypic evidence of eastern Aleutian intergrades).
These levels of gene flow are similar to earlier estimates (Peters et al. 2012)
and are well above levels deemed concordant with the ‘essentially
reproductively isolated’ criterion of the biological species concept (reviewed
by Winker 2021). Interestingly, divergence with gene flow was found in all
pairwise comparisons in this study, and three geographic modes of divergence
seem to be involved: parapatric (between crecca
and carolinensis; Peters et al.
2012), heteropatric (between crecca
and nimia;
Winker et al. 2013), and (mostly) allopatric (between carolinensis and ‘flavirostris’;
Spaulding et al. 2023). (Fun side note: Spaulding et al. (2023) hypothesized
that the small levels of gene flow between carolinensis
and ‘flavirostris’ result from
occasional re-colonization of South America by wintering carolinensis, preventing strict allopatry from occurring.)
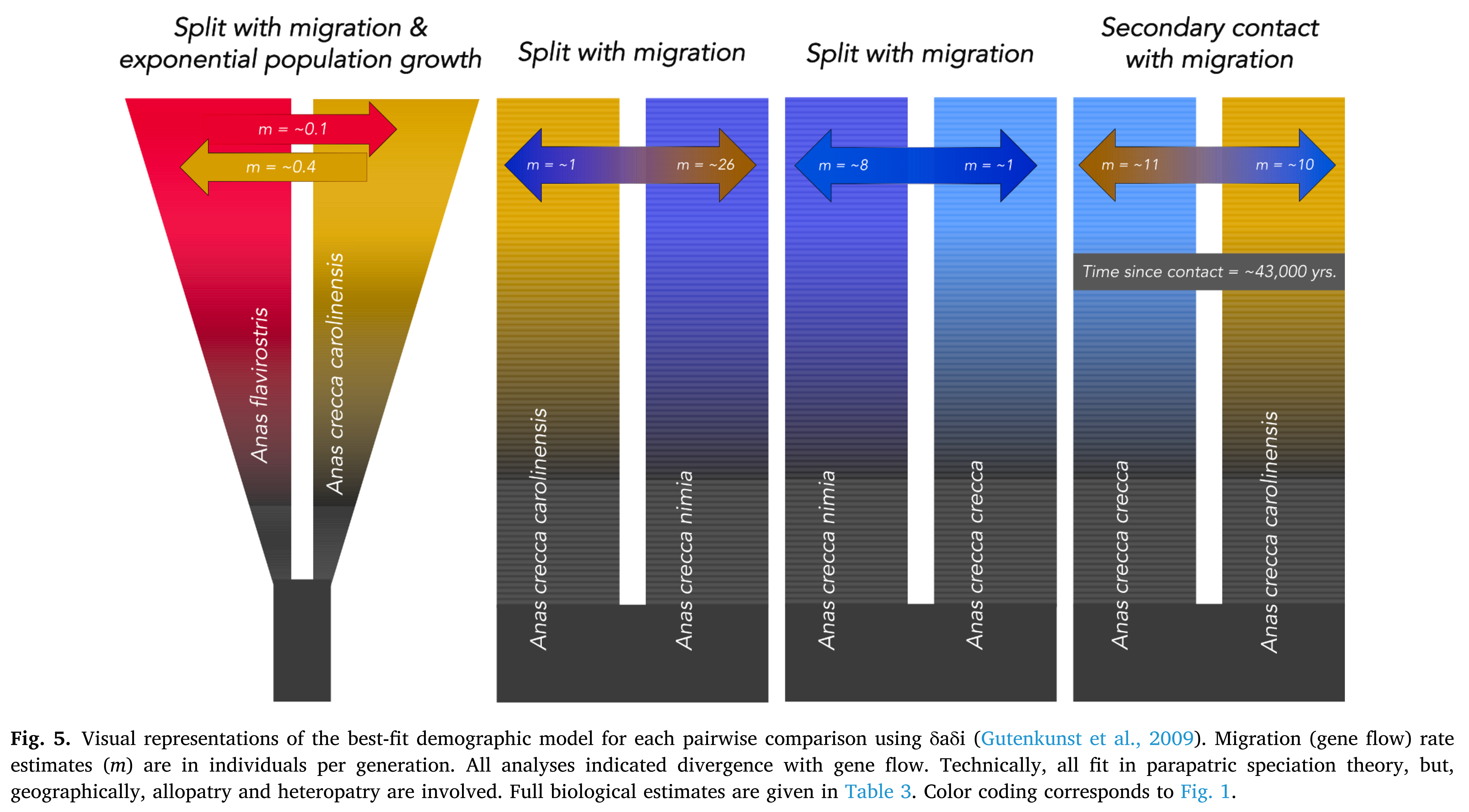
Spaulding et al. (2023: fig. 5). Best-fit demographic models of
pairwise population histories and corresponding estimates of gene flow using
UCEs. These levels of gene flow are entirely commensurate with current views of
species limits by NACC (Chesser et al. 2023).
Spaulding et al.’s
(2023) results using whole mitogenomes corroborated prior evidence (Johnson
& Sorenson 1999) of mtDNA paraphyly in the biological species of crecca+carolinensis: (crecca(carolinensis,’flavirostris’)). But, almost certainly because of
ongoing or cyclic gene flow, the nuclear relationship is quite strongly ((crecca,carolinensis),’flavirostris’) (Spaulding et al.
(2023:fig. 2, copied below). This situation thus seems to be a textbook example
of Haffer’s (1992:fig. 3, copied above) model of
speciation through ‘budding’. The currently favored hypothesis for this
situation is that mtDNA accurately tracks the group’s biogeographic
history―i.e., after Eurasia and North America were occupied at the onset of the
Pleistocene (~2.6 Mya) and mtDNA divergence between these populations was well
established, ancestors of ‘flavirostris’
colonized South America from North America and with considerable isolation this
population became its own well-differentiated biological species and has
continued to differentiate. But ongoing or intermittent gene flow between
Northern Hemisphere populations has prevented speciation between crecca and carolinensis (Johnson & Sorenson 1999, Spaulding et al. 2023).
It must be remembered
that these gene flow estimates are based on long-term effective population
sizes (Ne), and that such
population sizes are generally much lower than census size―especially among
higher-latitude species during our current interglacial period. Although these
values of gene flow provide key evidence about divergence in evolutionary time,
they are unlikely to be accurate with respect to on-the-ground evidence of
hybridization in today’s populations. Spaulding et al. (2022) used gene flow as
a proxy for levels of intercontinental movement in ducks as avian influenza
vectors and scaled these ‘evolutionary time’ values to today’s census sizes.
For A. crecca (sensu lato), it was
estimated that at present ~127 Eurasian-origin birds were likely to occur in
North America per teal generation. Considering that this is a
hybridization-based estimate, it seems noteworthy that it is roughly on par
with the eBird records of hybrids illustrated above (e.g., at an
order-of-magnitude level).
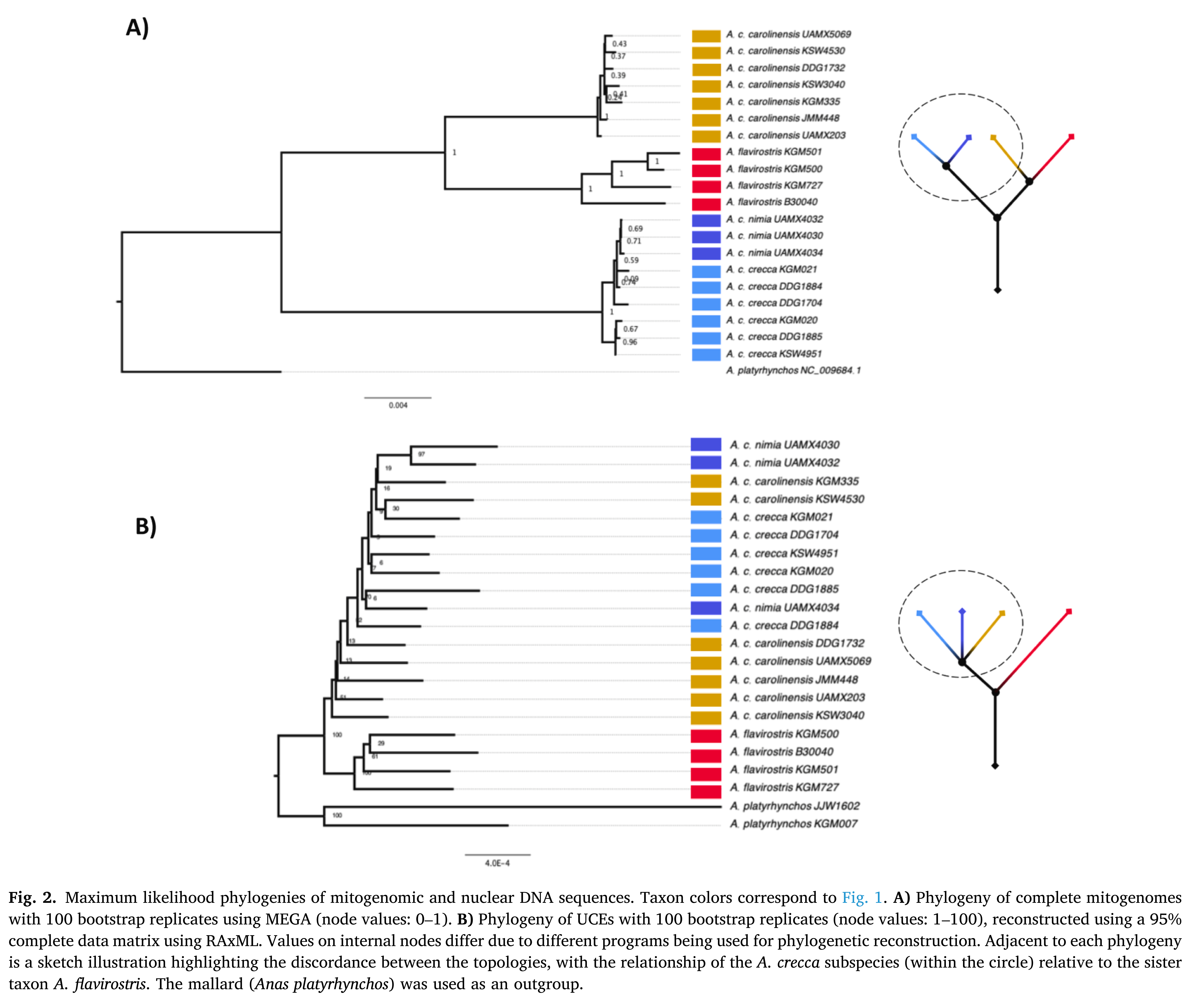
Spaulding et al. (2023:
fig. 2). Note the similarity of the sketched tree at upper right with Haffer’s (1992: fig. 3) model of speciation by ‘budding’.
Peters
et al. (2012) considered that female mate choice and migratory behavior
probably both cause some limitation of gene flow between crecca and carolinensis,
perhaps coupled with divergent selection. Sexual selection probably contributes
to male plumage differences and to the frequency differences found in male
displays (Laurie-Ahlberg & McKinney 1979).
Migratory direction could also cause some divergent selection if it has a
strong genetic component―although this seems less likely, because they migrate in
flocks, their movements are temporally variable, and they lack pronounced
winter site fidelity (Johnson 1995). It is not known whether there is
assortative mating when the two groups come into contact. But that is not a
particularly informative characteristic, given that it is commonly exhibited
within species and that premating isolating mechanisms are frequently
ineffective barriers upon secondary contact (especially relative to postmating
mechanisms; Irwin 2020). As Winker (2021:10) observed, “...neither the presence
nor the degree of assortative mating appears to be a reliable indicator of
species limits, either in birds or in other taxa in which it has been
studied." Peters et al. (2012:11) concluded that between these two taxa
“…the strength of divergent selection and ⁄ or the number of traits undergoing
such selection appear to fall short of that required for completion of
speciation given the estimated levels of nuclear gene flow."
This case offers a good opportunity to consider how we can
misread data to delineate biological species that do not meet the central BSC
criterion of being essentially reproductively isolated. It shows how diagnosability,
distinctiveness, and mtDNA relationships can be misleading and fail to properly
delimit species under the BSC. Here, the presence
of diagnostic adult male plumage traits and significant differences in male
courtship displays, coupled with mtDNA evidence of divergence and lack of
monophyly, drove decisions to split A.
crecca and A. carolinensis.
Although the presence of hybrids was recognized, the importance of
hybridization was diminished. For example, Sangster et al. (2001) stated that
“males showing a combination of characters of crecca and carolinensis…are
not evidence of a lack of reproductive isolation.” HBW-BirdLife (2020) and the
Tobias et al. (2010) methodology also diminished evidence of hybridization
(Winker 2010). When gene flow is evident, its extent is what is critical;
reproductive isolation is not an all-or-none phenomenon. Isolating mechanisms
are often incomplete, gene flow is common across step clines in birds, and this
case in teal shows how effective this gene flow can be in preventing
species-level divergence from occurring for long periods of time.
Taxonomy and nomenclature:
English names: If we
were to support a split, we would likely revert to the names used before the
two were lumped in 1973: Common Teal and Green-winged Teal (AOU 1957).
Recommendation:
Given considerable evidence from both male phenotype and diverse
genetic markers of substantial levels of gene flow between crecca and carolinensis,
these taxa are not biological species. Historic decisions under the BSC to
split these taxa recognized divergence in male phenotype and mtDNA, but did not
give evidence of hybridization sufficient weight. There are numerous issues yet
to be resolved about evolutionary divergence among members of the A. crecca-carolinensis-‘flavirostris’
clade, but finding essential reproductive isolation between crecca and carolinensis is not one of them. I recommend voting “No” on this
proposal.
Please vote yes (split into two species) or no (retain our
current taxonomy, recognizing a single biological species with two subspecies).
Literature Cited:
American
Ornithologists’ Union (AOU). 1973. Thirty-second supplement to the American
Ornithologists’ Union’s Check-list of
North American Birds. Auk 90:411-419.
American
Ornithologists’ Union (AOU). 1957. Check-list
of North American Birds, Fifth edition. American Ornithologists’ Union,
Baltimore, Maryland.
American
Ornithologists’ Union (AOU). 1983. Check-list
of North American Birds, Sixth edition. American Ornithologists’ Union,
Lawrence, Kansas.
Chesser, R. T., S. M. Billerman, K. J. Burns, C. Cicero, J. L. Dunn,
B. E. Hernández-Baños, R. A. Jiménez, A. W. Kratter, N. A. Mason, P. C.
Rasmussen, J. V. Remsen, Jr., and K. Winker. 2023. Check-list of North American
Birds (online). American Ornithological Society. https://checklist.americanornithology.org/taxa/
Cruickshank, A. D.
1936. Some observations of the European Teal. Auk 53:321-322.
Delacour, J., and E.
Mayr. 1945. The family Anatidae. Wilson Bulletin 57:1-55.
DeCicco, L. 2008.
Wildlife Observations at St. Paul Island, Alaska in 2008: U.S. Fish and
Wildlife Service Report, AMNWR 08/20. 21 pp.
del Hoyo, J. A.
Elliott, and J. Sargatal (eds.). 1992. Handbook
of the Birds of the World. Vol. 1 Lynx Edicions, Barcelona.
del Hoyo, J.; Collar, N. J.; Christie, D. A.;
Elliott, A.; Fishpool, L. D. C. 2014. HBW and BirdLife International
Illustrated Checklist of the Birds of the World. Volume 1: Non-passerines.
Lynx Edicions and BirdLife International, Barcelona, Spain and Cambridge, UK.
Felsenstein, J. 2005. Accuracy of coalescent
likelihood estimates: do we need more sites, more sequences, or more loci?
Molecular Biology and Evolution, 23(3), 691–700.
Funk, D. J., and K. E.
Omland. 2003. Species-level paraphyly and polyphyly: Frequency, causes, and
consequences, with insights from animal mitochondrial DNA. Annual Review of
Ecology, Evolution, and Systematics 34:397-423.
Gabrielson,
I. N. & Lincoln, F. C. 1959. Birds of
Alaska. Stackpole Company, Harrisburg, Pennsylvania.
Gavrilets, S. 2004. Fitness Landscapes and the Origin of Species.
Princeton Monographs in Population Biology No. 41.
Gibson, D. D., and G.
V. Byrd. 2007. Birds of the Aleutian
Islands, Alaska. Nuttall Ornithological Club and The American
Ornithologists’ Union, Cambridge, MA and Washington, D. C.
Gibson, D. D., and J.
J. Withrow. 2015. Inventory of the species and subspecies of Alaska birds,
second edition. Western Birds 46:94-185.
Gill, F., and M.
Wright. 2006. Birds of the World,
Recommended English Names – Version 1.0. Princeton, NJ, and London, UK:
Princeton University Press.
Haffer, J. 1992. The history of species concepts and species limits in
ornithology. Pp. 107-158 in Monk, J.
F., ed., Avian Systematics and Taxonomy.
Bull British Ornithological Club Centenary, Suppl. 112A.
Haffer, J. 2007. Ornithology, Evolution, and
Philosophy: The Life and Science of Ernst Mayr 1904-–2005. Springer, Berlin.
Hanna, G. D. 1920. New
and interesting records of Pribilof Island birds. Condor 22:173-175.
HBW and BirdLife
Taxonomic Checklist v5 (HBW-BirdLife). 2020. http://datazone.birdlife.org/species/taxonomy (retrieved 27 Sep
2023). http://datazone.birdlife.org/species/factsheet/common-teal-anas-crecca/details
Humphries, E. M., and
K. Winker. 2011. Discord reigns among nuclear, mitochondrial, and phenotypic
estimates of divergence in nine lineages of trans-Beringian birds. Molecular
Ecology 20:573-583.
Irwin, D. E. 2020. Assortative mating in hybrid zones is remarkably
ineffective in promoting speciation. American Naturalist 195:E150-E167.
Johnsgard, P. A. 1965. Handbook of Waterfowl Behavior. Comstock
Publishing Associates, Ithaca, NY.
Johnson, K. 1995.
Green-winged Teal (Anas crecca). In:
The Birds of North America Online (A. Poole, ed.). Cornell Lab of Ornithology,
Ithaca.
Johnson, K., and M. D.
Sorenson. 1999. Phylogeny and biogeography of dabbling ducks (genus Anas): a
comparison of molecular and morphological evidence. Auk 116:195–208.
Kessel, B. 1989. Birds of the Seward Peninsula, Alaska. University
of Alaska Press, Fairbanks, Alaska.
Konyukhov, N. B. 2015. [Rare and
vagrant birds of the Chukchi Peninsula.] Russian Journal of Ornithology 24
(express issue 1172):2717-2720.
Laurie-Ahlberg, C. C., and F. McKinney. 1979. The nod-swim display
of male Green-winged Teal (Anas crecca).
Animal Behaviour 27:165–172.
Lehman, P. E. 2019. The Birds of Gambell and St. Lawrence
Island, Alaska. Studies of Western Birds 4. Western Field Ornithologists,
Camarillo, California.
Mayr, E. 1940. Speciation phenomena in birds.
American Naturalist 74, 249-–278.
Mayr, E. 1942. Systematics
and the Origin of Species. New York, Columbia University Press.
Mayr, E., and L. L.
Short. 1970. Species taxa of North American birds. Publications of the Nuttall
Ornithological Club 9:1-127.
McLaughlin, J. F., and
K. Winker. 2020. An empirical examination of sample size effects on population
demographic estimates in birds using single nucleotide polymorphism (SNP) data.
PeerJ 8:e9939
Murie, O. J. 1959. Fauna of
the Aleutian Islands and Alaska Peninsula. North American Fauna 61:i-xiii,
1-406.
Palmer, R. S. 1976. Handbook of North American Birds, Vol.
2. New haven and London, Yale University Press.
Peters, J. L. 1931. Check-list of Birds of the World, Vol.
I. Harvard University Press, Cambridge.
Peters, J., K.
McCracken, C. Pruett, S. Rohwer, S. Drovetski, Y. Zhuravlev,
I. Kulikova, D. D. Gibson, and K. Winker. 2012. A
parapatric propensity for breeding in teal (Anas crecca, sensu lato) precludes
the completion of speciation. Molecular Ecology 21:4563-4577.
Poole, E. L. 1940.
Recent records from Lake Ontelaunee, Pennsylvania.
Auk 57:577-578.
Reeber, S. 2015. Waterfowl of North America, Europe, and
Asia: An Identification Guide. Princeton University Press, Princeton and
Oxford.
Remsen, J. V., Jr.
2010. Subspecies as a meaningful taxonomic rank in avian classification.
Ornithological Monographs 67:62-78.
Remsen, J. V., Jr.
2015. [Review of] HBW and BirdLife
International Illustrated Checklist of the Birds of the World, Vol. 1:
Non-passerines. Journal of Field Ornithology 86:182-187.
Remsen, J. V., Jr., J.
I. Areta, E. Bonaccorso, S. Claramunt, G. Del-Rio, A. Jaramillo, D. F. Lane, M.
B. Robbins, F. G. Stiles, and K. J. Zimmer. Version 28 Sep 2023. A
classification of the bird species of South America. Museum of Natural Science,
Louisiana State University. http://www.museum.lsu.edu/~Remsen/SACCBaseline.htm
Sangster, G., M.
Collinson, A. J. Helbig, A. G. Knox, D. T. Parkin, and T. Prater. 2001. The
taxonomic status of Green-winged Teal Anas
carolinensis. British Birds 94:218–226.
Sangster, G., A. G.
Knox, A. J. Helbig, and D. T. Parkin. 2002. Taxonomic recommendations for
European birds. Ibis 144:153–159.
Sibley, D. 2011.
Distinguishing Green-winged and Common Teal. https://www.sibleyguides.com/2011/03/distinguishing-green-winged-and-common-teal/ (retrieved 4 Oct 2023)
Spaulding, F. R., J. F.
McLaughlin, T. C. Glenn, and K. Winker. 2022. Estimating movement rates between
Eurasian and North American birds that are vectors of avian influenza (AI).
Avian Diseases 66:155-164.
Spaulding, F. R., J. F.
McLaughlin, K. G. McCracken, T. C. Glenn, and K. Winker. 2023. Population
genomics indicate three different modes of divergence and speciation with gene
flow in the green-winged teal duck complex. Molecular Phylogenetics and Evolution
182:000-000.
Tobias, J.A., N.
Seddon, C. N. Spottiswoode, J. D. Pilgrim, L. D. C. Fishpool, and N. J. Collar.
2010. Quantitative criteria for species delimitation. Ibis 152:724–746.
Vinicombe, K. E. 1994. Common
Teals showing mixed characters of Eurasian and North American races. British
Birds 87:88-89.
White, M.J.D. 1978. Modes of Speciation. W. H. Freeman and Company, San Francisco.
Winker, K. 2010. Is it
a species? Ibis 152:679-682.
Winker, K. 2021. An
overview of speciation and species limits in birds. Ornithology 138: ukab006
1-27.
Winker, K., K. G.
McCracken, D. D. Gibson, and J. L. Peters. 2013. Heteropatric speciation in a
duck, Anas crecca. Molecular Ecology
22:5922-5935.
Zink, R. M., S. Rohwer,
A. Andreev, and D. Dittmann. 1995. Trans-Beringia comparisons of mitochondrial
DNA differentiation in birds. Condor 97:639-649.
Kevin Winker, November 2023
Comments
from Zimmer:
“NO. For all of the reasons stated in this exceptionally thorough Proposal, and
reinforced personally, by 40 years of annual spring/summer visits to various
western Alaskan outposts (St. Paul Island, St. Lawrence Island, Adak),
particularly the Pribilofs, where obvious intergrade/hybrid male phenotypes are
not only regularly encountered, but outnumber phenotypically “classic” crecca
and carolinensis. Gene flow
between the two taxa is not only occurring, but is extensive in scope.”
Comments
from Remsen:
“NO for all the reasons in the proposal in addition to the apparent prevalence
of intermediate birds in the contact zone.
Evidently the plumage difference, although striking in one respect with
respect to the angle of the white “slash”, do not make a difference to the bird
in terms of mate selection.”
Comments from Robbins: “NO. Wow, this proposal is
indeed impressive in the amount of detail and in examining what the data
actually indicate. I vote NO, for the reasons Kevin has underscored in this proposal.”
Comments from Lane: “NO (for all the reasons outlined
in the proposal).”
Comments from Areta: “NO. In my mind, this has always
been a borderline case, and the impressive, nicely written, and thorough
proposal by Kevin shows that crecca
and carolinensis
are better treated as a single species on account of the nuclear
gene flow, the natural-history explanation of the mito-nuclear
discordance, the geographic breadth and frequency of hybridization, and the
minor plumage distinctions.”
Comments from Bonaccorso: “NO. From the offset, the plumage
differences seemed too subtle, but Kevin´s detailed explanation went well
beyond. Thanks Kevin for this impressive proposal. Indeed, understanding mito-nuclear discrepancies would be easier if we knew more
about which sex disperses in all birds. Also, many thanks to Kevin (Zimmer) for
the first-hand information about the abundance of hybrids.”
Comments from Claramunt:
“NO. The evidence presented in the proposal is convincing.”
Comments
from Stiles:
“NO; the amount of hybridization between these, wherever they come into
contact, is simply too frequent to justify this split.”