Proposal (995) to South
American Classification Committee
(Note from Remsen: This proposal was submitted to the North American
Classification Committee and passed, and it is submitted here with permission
from Eric VanderWerf. Note that brewsteri,
as represented by the subspecies etesiaca, breeds in the SACC area on
Isla Gorgona, Colombia.)
Treat Sula brewsteri as a
separate species from Brown Booby S.
leucogaster (Part A), and if Part
A passes, establish English name for Sula
brewsteri (Part B)
Part A.
Background:
Note: much
of the background information below is from VanderWerf et al. (2023)
The Brown Booby (Sula leucogaster) is a pantropical seabird found in the Pacific,
Atlantic, and Indian Oceans that exhibits geographic morphological variation.
Five subspecies have been described based on differences in color of the
plumage, bill, and facial skin (Table 1; Nelson 1978, VanderWerf 2018a,
Schreiber & Norton 2020). Sula l.
plotus has the largest geographic range, from the Red Sea and Indian Ocean
east to the central Pacific. In both sexes of S. l. plotus, the head is
dark brown, and the bill is yellow (females) or bluish-yellow (males). The
nominate subspecies, S. l. leucogaster,
occurs in the Atlantic and Caribbean and is similar to plotus but has a more pinkish bill. The form of Brown Booby
occurring in the eastern Pacific, S. l.
brewsteri, is the most distinctive morphologically and originally was
described as a separate species called Brewster’s Booby (S. brewsteri; Goss 1888). Male S.
l. brewsteri have a geographically variable white head, a more grayish-blue
bill, and a more greenish-blue gular pouch. In the Gulf of California and the
western coast of Mexico south to the Revillagigedo Islands, male S. l. brewsteri have a white head and a
pale brown upper neck. On some islands off Central America and South America,
the white on the head of males is restricted to the forehead, and this form
sometimes is considered a separate subspecies, S. l. etesiaca, but also has been grouped with S. l. brewsteri (Harrison 1983, Schreiber & Norton 2020). The
form breeding on Clipperton Island is the palest, with the entire head and neck
white in males, and is sometimes referred to as S. l. nesiotes (Heller and Snodgrass 1901, Pitman & Balance
2002, Schreiber & Norton 2020).
As noted above, Brewster’s Booby originally was
described as a species, S. brewsteri,
by Goss (1888) based on type specimens from San Pedro Mártir Island, Mexico. In
the description, Goss noted that characteristics of the species included the
pale color of the head and neck, especially in the male, a dark brown iris with
a narrow outer ring of grayish-white, and “unfeathered parts also differently
colored.” In 1944, S. l. brewsteri was lumped with other forms of
the Brown Booby (Wetmore et al. 1944), who cited “Peters, checklist, 1, 1931”
and Wetmore (1939) as justification for the lump. Wetmore (1939) stated that
“while apparently uniform over large areas of the tropical oceans of the world
there are three (barely possibly four) races of Sula leucogaster to be recognized…on the Pacific coast of the
Americas… These differ from all other subspecies of the species.” Wetmore
(1939) discusses at length the variation among the forms in the eastern
Pacific, which seems to support their distinction, and did not offer any
explanation for why S. l. brewsteri
should be lumped with other forms of the Brown Booby, yet he did so, and this,
paradoxically, served as the basis for the lumping by Wetmore et al (1944).
Genetic population structure of Brown Boobies
largely matches patterns of morphological variation (Steeves et al. 2003,
Morris-Pocock et al. 2010, 2011). Mitochondrial haplotypes were not shared
between the eastern and central Pacific or between the eastern Pacific and
Caribbean (Steeves et al. 2003), and colonies grouped into four major,
genetically differentiated populations; Caribbean, central Atlantic,
Indo-central Pacific, and eastern Pacific (Morris-Pocock et al. 2011). The
eastern Pacific population was found to be the most different genetically and
was estimated to have diverged from all other populations approximately one
million years ago (Morris-Pocock et al. 2011). These populations have diverged
because of a combination of physical barriers (the Isthmus of Panama and the
Eastern Pacific Basin) and a behavioral tendency in the Brown Booby to forage
closer to shore than other booby species (Steeves et al. 2003).
Table 1. Distinguishing characteristics of Brown Booby subspecies.
Copied from VanderWerf et al. (2023).
|
Character |
plotus |
leucogaster |
brewsteri |
nesiotes |
etesiaca |
|
Male
head color |
brown |
brown |
white
head and upper neck |
white
head and entire neck |
white
forehead |
|
Female
head color |
brown |
brown |
whitish
forehead |
whitish
forehead |
whitish
forehead |
|
Male
bill color |
bluish-yellow |
bluish-yellow |
grayish-blue |
grayish-blue |
grayish-blue |
|
Female
bill color |
yellow |
pinkish
yellow |
pinkish
yellow |
pinkish
yellow |
pinkish
yellow |
|
Ventral lesser wing coverts |
white |
white |
white
with brown bar |
white
with brown bar |
? |
New Information:
Three types of new information support
recognizing Brewster’s Booby as a separate species: 1) morphological data
demonstrating that males and females of the subspecies differ in additional
ways not previously recognized; 2) behavioral data on pairing patterns showing
that interbreeding between plotus and
brewsteri is rare despite increasing
sympatry; 3) information on behavioral ecology demonstrating that the
morphological differences between plotus
and brewsteri act as a reproductive
isolating mechanism that inhibits interbreeding. The information on behavioral
ecology is not that new, but its relevance to taxonomy does not seem to have
been considered previously.
1. Morphology. Descriptions of Brown Booby subspecies relied
primarily on the appearance of males and did not consider the appearance of
females. VanderWerf (2018) described differences between females of the
subspecies that can be used to identify them in the field: female S. l. brewsteri have a pinker bill and
paler forehead than female S. l. plotus.
Females can be distinguished as reliably as males of these subspecies, although
the characters used to identify females are less obvious. VanderWerf (2018) also
showed that the underwing coverts in both sexes of S. l. brewsteri are less extensively white than those of S. l. plotus.
I have unpublished data showing that iris color
and bill curvature also differ among Brown Booby subspecies, but I am still in
the process of collecting and analyzing these data. I do not think this
information about additional distinguishing characters is needed to assess the
taxonomy of Brown Boobies, but it will be useful in their identification.
2. Pairing Patterns. The Eastern Pacific Basin is
an enormous, island-free ocean area that for millennia has formed a physical
barrier to dispersal and promoted geographic differentiation of many seabirds,
including the Brown Booby (Avise et al. 2000, Steeves et al. 2003, Morris-Pocock
et al. 2011). Recently, Brown Boobies have been overcoming the barrier posed by
the Eastern Pacific Basin and have dispersed eastward and westward across the
Pacific. VanderWerf et al. (2008) documented an increasing number of S. l. brewsteri present and breeding in
the central Pacific, and Kohno and Mizutani (2011)
documented the occurrence and breeding of S.
l. brewsteri males on islands near Japan. Isla San Benedicto,
in the Revillagigedo Islands off the west coast of Mexico, was recolonized by
Brown Boobies following a volcanic eruption in 1952, and both plotus and brewsteri males are present and breeding on the island (Pitman and
Ballance 2002, Morris-Pocock et al. 2011). VanderWerf et al. (2023) showed that
the westward expansion of brewsteri
has continued, resulting in even greater sympatry between the subspecies. The
increasing sympatry of S. l. brewsteri
and S. l. plotus could result in gene
flow and erosion of differentiation between these forms if they interbreed.
VanderWerf et al. (2023) also collected data on
pairing patterns in locations where both forms were known to breed together.
Quantitative data showed pairing by S. l.
brewsteri and S. l. plotus was
primarily assortative and interbreeding was rare (Table 2). At Moku Mana, Maui,
in 2021, there were fewer mixed pairs (zero), than expected by chance (X2
= 18.00, df = 1, p < 0.001). On Palmyra, there also were fewer mixed pairs
than expected by chance (X2 = 181.1, df = 1, p < 0.001), although
the sample size was small.
Anecdotal evidence also indicates that pairing
was primarily assortative, and that interbreeding was rare. On Wake Island in
2021, J. Gilardi observed 301 S. l. plotus pairs, six S. l.
brewsteri males, and one S. l.
brewsteri female, which was paired with one of the male S. l. brewsteri. The other five male S. l. brewsteri on Wake that year did
not attract a mate despite the presence of many unpaired S. l. plotus females. On Wake in 2022, J. Gilardi
observed that the brewsteri-brewsteri pair remained together and
raised another chick, but the other five brewsteri
males were unpaired. On Wake in 2023, J. Gilardi
observed seven male and four female brewsteri;
all 4 brewsteri females were paired
with brewsteri males, and the three
single brewsteri males built nests
but had no mate. On Moku Manu, Oahu, in May 2021, E. VanderWerf observed 93 S. l. plotus pairs and only one male and
one female S. l. brewsteri, which
were paired with each other and had a large chick. In September 2022, E.
VanderWerf observed five S. l. brewsteri
females on Moku Manu, all of which were unpaired. On Laysan, a nest with a male
brewsteri and female plotus was reported in 1998 (VanderWerf
et al. 2008), but re-examination of photos using identification criteria from
VanderWerf (2018) revealed that the female was S. l. brewsteri, representing another instance in which a male and
female brewsteri paired with each
other amid large numbers of male and female plotus.
The only instances of interbreeding occurred in locations where no female brewsteri
were present. On Midway, J. Plissner observed a male brewsteri x female plotus pair that raised a chick in 2020, when no female brewsteri were present. At Nakanokamishima Island, Japan, one brewsteri male paired and raised offspring with a plotus female from 2012-2014, but a
second brewsteri male was not able to
attract a mate despite frequent courtship attempts with plotus females (Kohno and Mizutani 2015).
Moku Mana Islet off the north coast of Maui is a
particularly interesting case. It was colonized by Brown Boobies recently, with
the first nest documented in 2004 by J. Penniman. In 2021, the island held
twice as many S. l. brewsteri pairs
as S. l. plotus pairs, with no mixed
pairs. This is the easternmost location in the Hawaiian Islands where Brown
Boobies breed, and the colonies were formed by individuals dispersing from the
east (S. l. brewsteri) and west (S. l. plotus), much like the situation
on Isla San Benedicto (Pitman and Ballance 2002).
Table 2. Pairing patterns of Brown Boobies by
subspecies, including only cases in which the identity of both parents was
known. Copied from VanderWerf et al. (2023).
|
Location |
Year |
Male
plotus + female plotus |
Male
plotus + female brewsteri |
Male brewsteri
+ female plotus |
Male brewsteri
+ female brewsteri |
|
Palmyra
Atoll † |
2014 |
~200 |
1 |
0 |
8 |
|
Moku
Mana |
2021 |
6 |
0 |
0 |
12 |
|
Moku
Manu |
2021 |
93 |
0 |
0 |
1 |
|
Laysan |
1998 |
~70 |
0 |
0 |
1 |
|
Midway ‡ |
2020 |
15 |
NA |
1 |
NA |
|
Wake
Island |
2023 |
301 |
0 |
0 |
4 |
|
Nakanokamishima ‡ |
2009 |
~900 |
NA |
1 |
NA |
†
At Palmyra 30 nests were observed with at least one brewsteri parent, but some nests were attended by a single parent
and the identity of the mate was unknown.
‡ At Midway and Nakanokamishima
no female brewsteri were
present so there was no chance of a mixed pair.
3. Reproductive Isolating Mechanisms. Mate
choice and breeding biology of Brewster’s Booby have been studied extensively
in Mexico, and this literature is important for understanding the pairing
patterns observed in the central Pacific. Most importantly, López-Rull et al. (2016) showed that male S. l. brewsteri with their head painted brown to look like male S. l.
plotus were treated aggressively by their mate and that the level of
aggression was higher at a colony closer to the zone of overlap between the
forms. They concluded that female dislike of foreign males may function as a
reproductive barrier in populations close to contact zones, where the risk of
possibly maladaptive hybridization is highest. Montoya et al. (2018) showed
that the carotenoid-based greenish-blue color of the gular pouch of males was
energetically expensive to maintain, that its chroma peaked during courtship,
and that it may serve as a reliable signal of individual quality. Michael et
al. (2018) showed that color of the gular pouch in Brown Boobies in México was
related to foraging range and location, with individuals in poor body condition
constrained to low-cost, short-distance foraging trips closer to shore, where
they were unable to obtain the pelagic diet necessary for production of the
carotenoid-rich gular pouch ornament important in mate attraction.
Cumulatively, this research indicates that the morphological differences
between S. l. brewsteri and S. l. plotus act as an isolating
mechanism that inhibits interbreeding.
The morphological
and genetic differences between S. l.
brewsteri and other forms of the Brown Booby meet the standards for species
recognition under the typological (or morphological) and phylogenetic species
concepts, respectively (Mayr 2000, Wheeler 2000). The behavioral evidence
described in this study, increasing sympatry with primarily assortative mating
and rare interbreeding, meets the standards of the biological species concept
(Mayr 2000). Cumulatively, all three forms of evidence suggest that it would be
appropriate to consider Brewster’s Booby as a separate species again.
Occasional hybridization between S. l. brewsteri and S. l. plotus and the presence of some individuals of intermediate
appearance do not constitute evidence that they are conspecific. Similar
situations exist in two other pairs of booby species, specifically Blue-footed
Booby (S. nebouxii) and Peruvian
Booby (S. variegata), and Masked
Booby (S. dactylatra) and Nazca Booby
(S. granti). Blue-footed and Peruvian
boobies are largely allopatric, but breeding colonies occur together on two
islands off Peru, where mating is primarily assortative, with few instances of
hybridization, resulting in genetic differentiation and only limited introgression
(Figueroa 2004, Figueroa and Stucchi 2008, Taylor et al. 2012). Nazca Booby was
considered a subspecies of Masked Booby but was split into a separate species
based on morphological differences and assortative mating (Pitman and Jehl
1998, AOU 2000), which subsequently was supported by evidence of genetic
differentiation (Friesen et al. 2002).
Part B. English name:
For the English common name, according to NACC
guidelines for English names, when a split restores species status and a
previously existing English name exists, that name would be resurrected. In
this case the name would have been Brewster’s Booby. However, given the recent
decision by AOS to change all eponymous common bird names, the name Brewster’s
Booby is no longer an option. Below I have listed several possible alternative
English common names, grouped by whether they are geographical or descriptive of
the bird. I have offered some discussion of each name, including the pluses and
minuses. My personal favorite is Cocos Booby and I recommend that as the new
English common name for. S. brewsteri.
GEOGRAPHIC NAMES
Cocos Booby. The rationale for this name is similar to that
of the Nazca Booby, which is named for a tectonic plate in the earth’s crust
that contains part of the range of that species. The Cocos Plate is located in
the eastern Pacific and is adjacent to and north of the Nazca Plate. The Cocos
Plate extends off the west coast of the Americas from Panama north to the
Mexican state of Jalisco (Figure 1). The volcanoes and earthquakes in western
Mexico and Central America are fueled by plate convergence and subduction of
the Cocos Plate under the North American and Caribbean plates. The Cocos Plate
is named for Cocos Island, which rides upon the plate and is the only part of
the plate above sea level. Cocos Island is an important nesting site for S. brewsteri, although the colonies
there are not among the largest of the species. One downside to this name is
that the Cocos Plate encompasses only a small portion of the geographic range
of S. brewsteri, although it contains
a larger proportion of the range than the Nazca Plate contains of the Nazca
Booby range. The parallel naming of Nazca Booby and Cocos Booby would provide a
logical, geographical rationale for these two species that overlap considerably
in range.
Mexican Booby. The name Mexican Booby would recognize that
much of the range of S. brewsteri
occurs in Mexico and Mexican Waters and that the type specimens were collected
in Mexico. Two drawbacks to this name are that 1) two subspecies that would be
included with S. brewsteri (S. b. nesiotes and S. b. etesiaca) are not found in Mexico, and 2) increasingly less
of the range of S. brewsteri will be
in Mexico if the current range expansion continues across the Pacific. These
same drawbacks also pertain to the name Cocos Booby. The name Mexican Booby
would provide historical context for the likely origin for many of these
dispersing birds.
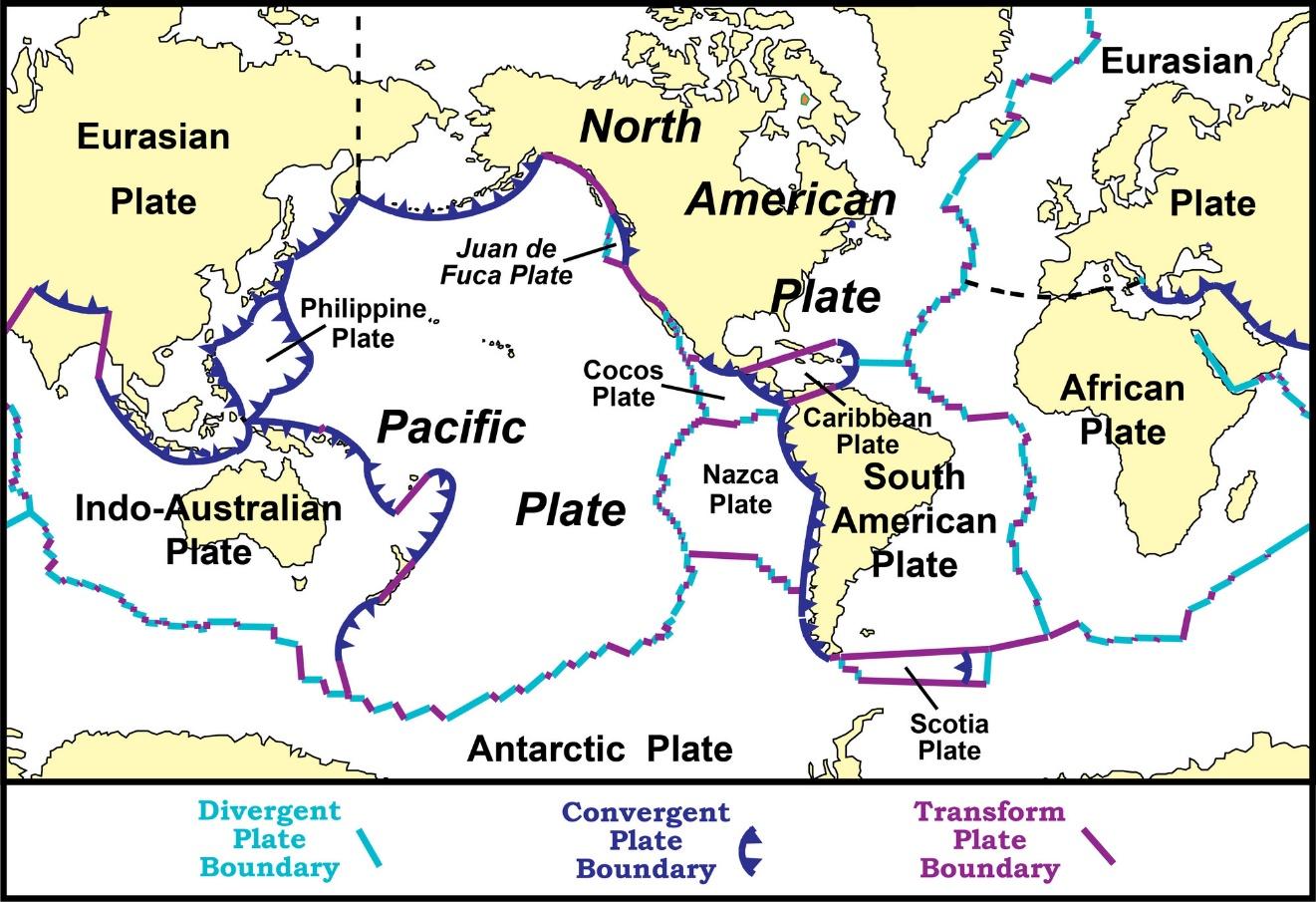
Figure 1. Map of the earth’s tectonic plates, showing the
location of the Cocos Plate. From National Park Service: https://www.nps.gov/subjects/geology/plate-tectonics-evidence-of-plate-motions.htm
Tropical Eastern Pacific Booby. The Tropical
Eastern Pacific is a name applied to one of twelve marine realms that cover the
coastal waters and continental shelves of
the world's oceans as part of a global classification system called Marine
Ecoregions of the World (MEOW), which was devised by an international team,
including conservation organizations, academic institutions and
intergovernmental organizations (Spalding et al. 2007). It extends along the
Pacific Coast of the Americas, from the
southern tip of the Baja California Peninsula
in the north to northern Peru in the
south. The range of S. brewsteri
largely coincides with this geographic unit, though the species range does
extend farther to the north and south. Among geographic names this would be the
most geographically appropriate because it coincides most the with range of the
species. The main downside to this name is that it is long and cumbersome,
consisting of four words.
DESCRIPTIVE NAMES
White-headed Booby. The name White-headed Booby
is based on the most distinctive character of the species, the white head of
males, which was the character that seems to have contributed most to its
description as a separate species. However, this name has two significant drawbacks
that in my opinion disqualify it: 1) it would pertain only to males of the brewsteri and nesiotes forms, and not to males of etesiaca; and 2) it would not pertain to females of any form, and
thus would perpetuate the neglect of female appearance that has occurred
already.
Pale-headed Booby. Pale-headed Booby would be
preferable to White-headed Booby because it would apply to females and males of
all forms that would be included in S.
brewsteri. The main downside is that it seems a little bland.
Recommendation:
I recommend that S. l. brewsteri be split from other forms of the Brown Booby into a
separate species. This split has been mentioned previously (Schreiber and
Norton 2020), and the additional evidence described in this proposal
strengthens the rationale for the split. The scientific name for the re-split
species would revert to S. brewsteri,
as it was originally described by Goss (1888). Sula brewsteri should include all pale-headed subspecies that breed
in the eastern Pacific, including nesiotes
and etesiaca recognized by some
authors (Schreiber and Norton 2020). If subspecific status continues to be
recognized for these forms, they would become S. brewsteri nesiotes and S.
brewsteri etesiaca, respectively.
Although etesiaca males have a
pale, not white, head, etesiaca
females are similar to females in other forms of brewsteri, and genetically etesiaca
clearly grouped with other populations in the eastern Pacific (Steeves et al.
2003, Morris-Pocock et al. 2010). No changes are needed in taxonomic status or
nomenclature of the other Brown Booby subspecies, which are more similar to
each other morphologically and genetically (Morris-Pocock et al. 2011). Thus, S. l. plotus and S. l. leucogaster would remain subspecies of the Brown Booby.
For the English name, I recommend Cocos Booby,
for the reasons discussed above.
Note from Remsen on voting on Part B. A YES vote is for Cocos Booby,
the recommendation of the proposal and the unanimous choice of NACC, and a NO
is for some other name; please indicate if that is Brewster’s Booby, the
traditional name of the taxon, or some novel name.
Literature Cited:
Avise, J. C., W. S.
Nelson, B. W. Bowen, and D. Walker. 2000. Phylogeography of colonially nesting
seabirds, with special reference to global matrilineal patterns in the sooty
tern (Sterna fuscata). Molecular
Ecology 9:1783-1792.
Del Hoyo, J., A. Elliott, and J. Sargatal. 1992. Handbook of birds of
the world. Volume 1. Barcelona: Lynx Editions.
Figueroa, J. 2004. First record of breeding by the Nazca Booby Sula granti on Lobos de Afuera Islands,
Peru. Marine Ornithology 32:117-118.
Figueroa, J., and M.
Stucchi. 2008. Possible hybridization between the Peruvian Booby Sula variegata and the Blue-footed Booby
S. nebouxii in Lobos de Afuera
Islands, Peru. Marine Ornithology 36:75-76.
Friesen, V. L., D. J. Anderson, T. E. Steeves, H. Jones, and E. A.
Schreiber. 2002. Molecular support for species status of the Nazca Booby (Sula granti). Auk 119:820–826.
Goss, N. S. 1888. New
and rare birds found breeding on the San Pedro Martir
Isle. Auk 5:240-244.
Heller, E. and Snodgrass, R.E. 1901. Descriptions of two new species and
three new subspecies of birds from the Eastern Pacific, collected by the
Hopkins-Stanford expedition to the Galapagos Islands. Condor:74-77.
Kohno, H., and A. Mizutani. 2011. The first
record of Brewster’s Brown Booby Sula
leucogaster brewsteri for Japan. J. Yamashina Institute for Ornithology
42:147‒153.
Kohno, H., and A. Mizutani. 2015. First record
of breeding behaviour of Brewster’s Brown Booby Sula leucogaster brewsteri in Japan. J. Yamashina
Institute for Ornithology 46:108-118.
López-Rull, I., N. Lifshitz, C. M. Garcia, J.
A. Graves, and R. Torres. 2016. Females of a polymorphic seabird dislike
foreign-looking males. Animal Behaviour 113:31-38.
Michael, N.P., R. Torres, A. J. Welch, J. Adams, M. E. Bonillas-Monge, J. Felis, L. Lopez-Marquez, A.
Martínez-Flores, and A. E. Wiley. 2018. Carotenoid-based skin ornaments reflect
foraging propensity in a seabird, Sula
leucogaster. Biology letters 14:20180398.
Montoya, B., C. Flores, and R. Torres. 2018. Repeatability of a dynamic
sexual trait: Skin color variation in the Brown Booby (Sula leucogaster). The Auk: Ornithological Advances 135:622-636.
Morris-Pocock, J. A., D. J. Anderson, and V. L. Friesen. 2011.
Mechanisms of global diversification in the Brown Booby (Sula leucogaster) revealed by uniting statistical phylogeographic
and multilocus phylogenetic methods. Molecular Ecology 20:2835‒2850.
Morris-Pocock, J. A., T. E. Steeves, F. A. Estela, D. J. Anderson, and
V. L. Friesen. 2010. Comparative phylogeography of Brown (Sula leucogaster) and Red-footed Boobies (S. sula): the influence of physical barriers and habitat preference
on gene flow in pelagic seabirds. Molecular Phylogenetic and Evolution
54:883‒896.
Nelson, J. B. 1978. The Sulidae. Oxford University Press, London.
Pitman, R. L., and L. T. Ballance. 2002.
The changing status of marine birds breeding at San Benedicto
Island, Mexico. Wilson Bulletin 114: 11-19.
Pitman, R., and J. Jehl. 1998. Geographic variation and reassessment of
species limits in the “Masked” boobies of the eastern Pacific Ocean. Wilson
Bulletin 110:155–170.
Rauzon, M. J., D. Boyle, W. T. Everett, and J. Gilardi.
2008. The status of birds of Wake Atoll.
Atoll Research Bulletin 561:1-41.
Schreiber, E. A., and R. L. Norton. 2020. Brown Booby (Sula leucogaster), version 1.0 In Birds
of the World (S.M. Billerman, Ed.). Cornell Lab of Ornithology, Ithaca, NY,
USA: https://doi.org/10.2173/bow.brnboo.01
Spalding, M.D., H. E. Fox, G.R. Allen, N. Davidson, et al. 2007. Marine
Ecoregions of the World: A Bioregionalization of Coastal and Shelf Areas.
Bioscience 57(7):573–583.
Steeves, T. E, D. J. Anderson, H. Mcnally, M.
H. Kim, and V. L. Friesen. 2003. Phylogeography of Sula: the role of physical barriers to gene flow in the
diversification of tropical seabirds. J. Avian Biology 34:217-223.
Steeves, T. E, D. J. Anderson, and V. L. Friesen. 2005. A role for
nonphysical barriers to gene flow in the diversification of a highly vagile
seabird, the Masked Booby (Sula
dactylatra). Molecular Ecology 14:3877-3887.
Taylor, S. A., D. J. Anderson, C. B. Zavalaga,
and V. L. Friesen. 2012. Evidence for strong assortative mating, limited gene
flow, and strong differentiation across the Blue‐footed/Peruvian
booby hybrid zone in northern Peru. Journal of Avian Biology 43:311-324.
VanderWerf, E. A.
2018a. Geographic variation in the Brown Booby. Field identification of males
and females by subspecies. Birding 50:48-54.
VanderWerf, E. A., B. L. Becker, J. Eijzenga,
and H. Eijzenga. 2008. Nazca Booby Sula granti and Brewster’s Brown Booby Sula leucogaster brewsteri in the
Hawaiian Islands and Johnston and Palmyra Atolls. Marine Ornithology 36:67–71.
VanderWerf, E. A., M.
Frye, J. Gilardi, J. Penniman, M. Rauzon,
H. D. Pratt, R. S. Steffy, and J. Plissner. 2023.
Range expansion, pairing patterns, and taxonomic status of Brewster’s Booby Sula leucogaster brewsteri. Pacific
Science 77:1-12.
Wetmore, A. 1939.
Birds from Clipperton Island collected on the presidential cruise of 1938.
Smithsonian Miscellaneous Collections 98(22):1-6.
Wetmore, A.,
Friedmann, H., Lincoln, F.C., Miller, A.H., Peters, J.L., van Rossem, A.J., Van
Tyne, J., and Zimmer, J.T. 1944. Nineteenth supplement to the American
Ornithologists’ Union Check-list of North American birds. Auk 61:441-464.
Wheeler, Q. D. 2000.
The phylogenetic species concept (sensu
Wheeler and Platnick). Pp. 55-60 in Species Concepts
and Phylogenetic Theory: a Debate (Q.D. Wheeler and
R. Meier, eds.).
Columbia University Press, New York.
Eric A. VanderWerf,
April 2024
Additional notes on Part B – English name. When Remsen told VanderWerf
that SACC was not bound by the edict of the AOS Council on removing all
eponyms, VanderWerf responded by noting that he was not opposed to eponyms but
still preferred Cocos Booby for the following reasons: “I
prefer Cocos Booby for 3 reasons: 1) I like geographic names, and
this offers a nice parallel with Nazca Booby; 2) if the NACC also adopts the
split, then there would be a chance that the same English name, Cocos Booby,
would be recognized by both groups. Having different common names would be
confusing; and 3) the Spanish name, which has not been discussed and I don’t
know where/how/when that would happen, presumably would be Bobo Cocos, which
has a nice ring to it.”
Comments
from Remsen:
“Part
A: YES. The lack of non-assortative mating is striking especially because
reproductive isolating mechanisms in birds tend to be weakest when range
expansions bring taxa together that have had little previous contact. Also, note that the phenotypic differences
between Sula dactylatra and Sula granti roughly comparable to
those between brewsteri and leucogaster. Note also that there was no evidence for the
Peters-Wetmore lump of the two species.
The 4th edition of the AOU Check-list (1931) treated it as a
separate species, Brewster’s Booby (S. brewsteri) from Sula l.
leucogaster (White-bellied Booby), and the broadly defined species
treatment then appeared in the 5th edition (1957) based solely on
Peters’ and Wetmore’s treatment.
“Part
B: NO. I think Cocos is a much better name than any of the others in the
proposal for the biogeographic rationale outlined therein. Nevertheless, the title of VanderWerf et
al.’s paper makes it clear why Brewster’s is the better name in that that is
the name that seabird people use for the distinctive taxa of the eastern
Pacific. What better evidence do we need
than this that the name has about 130+ years of formal and informal association
with the eastern Pacific taxa? See also
the title of VanderWerf et al. (2008) above.
See also any of the 20+ papers, most of them published in The Auk,
that used “Brewster’s Booby”, as revealed by Google Scholar. Google search on “Brewster’s Booby” produces
934 hits. Brewster’s Booby was the name
used by the AOU (1931), where treated as a separate species. Hellmayr and Conover (1948) also used
Brewster’s Booby for S. l. brewsteri.
More recently, Howell and Zufelt (Oceanic Birds of the World, 2019,
Princeton Univ. Press) used “Brewster’s Brown Booby” for S. l. brewsteri.
“Although Goss, who also described brewsteri,
made no attempt to justify that name and in fact did not make it clear that brewsteri
presumably honored “the” Brewster of ornithology, i.e. William Brewster. That was because it was so obvious that
William Brewster’s contributions to the avifauna of Baja California (e.g.,
“Birds of the Cape region of Lower California”, 1902, Bull. Museum Comp.
Zoology, 241 pp., and many additional papers, based on existing collections and
collections that he commissioned, but not on his own fieldwork) were so
important that no explanation was needed.
“William Brewster’s contributions to
ornithology go far beyond Baja California.
He was one of three founding members of the American Ornithological
Society itself (1873), and served as its president for three years. The AOS’s Brewster Medal is one of the three
highest awards it gives for research.
Brewster was a Fellow of the American Academy for Advancement of Science
(AAAS). He was the founder and a president of the Nuttall Ornithological Club,
the oldest ornithological society in the Western Hemisphere. He was the first president of Mass Audubon
from 1896 to 1913, and the major goal of that society was to restrict the
killing of wild birds and was a force behind passage of the Lacey Act of 1900
(which prohibits interstate commerce in wild birds killed illegally). Brewster was curator of birds at Harvard’s
Museum of Comparative Zoology from 1885 until his death in 1919. Henshaw’s 23 page memorial to Brewster in the Auk in 1920 outlines his many
additional contributions and personal attributes; Henshaw made it clear that Brewster was what
we would call a “good guy” nowadays:
‘He
was charitably disposed to all, and inclined to judge the delinquent leniently
and with forbearance. He never spoke ill of any man. He was generously
inclined, and, within his means, gave freely to those less fortunate than
himself, though of his beneficence he said nothing, preferring that it should remain unknown.
‘He
was absolutely truthful, habitually refrained from all exaggeration, and
falsehood and evasion were foreign to his nature. As he was sincere and
truthful, so was he honorable and pure minded, and his conversation reflected
the thoughts and imaginings of a pure soul.’

“Some tidbits gleaned from these and other
sources: All three of his siblings died in childhood; their early deaths
evidently inspired the poem The Open Window, by Henry Wadsworth
Longfellow, one of the Brewster family’s neighbors. Brewster had some sort of vision problem as a
child, but that seems to have passed as he became an adult. His extensive field notes have been digitized
and are online, and browsing through these, it is clear that Brewster was not
only a keen observer of birds but really loved them. Actually watching birds and describing their
behavior was his passion, and in this way seemed ahead of his time despite what
must have been primitive optics. Perhaps
in compensation, he had a reputation for extremely sharp hearing and ability to
identify birds at a distance by voice to the extent that Henshaw considered him
supremely talented:
‘His hearing was
extraordinarily acute, and his ability to recognize the notes of birds at a
distance and amid other and confusing sounds was little less than marvelous,
and far exceeded that of any one I ever knew.’
“His 38 years of fieldwork at Lake Umbagog, on the Maine-New Hampshire border, produced some
legendary observations, often from his canoe, to the point that Robert Stymiest published excerpts as a paper in the Bird Observer in 2004. At Lake Umbagog, he
made the first detailed observations
of the nesting behavior of the Philadelphia Vireo, as just one of his
many contributions. He was also one of
many famous naturalists who made extensive observations in the Concord,
Massachusetts, and kept copious field notes on his experiences there; in fact,
in 1937, a 300 page book of
selections from his Concord journals was published.
Brewster was also interested in bird migration, and actually spent 6
weeks in Fall 1885 at a lighthouse in the Bay of Fundy do study the behavior of
migrating birds. The paper he published
on these observations was a classic of the era.
“I herein take the opportunity to make some
positive remarks on eponymous English names.
Brewster’s case, in my opinion, provides a strong example of the value
of eponyms. Yes, many of these founders
of American ornithology are memorialized in other ways, including scientific
names. However, most birders and even
many professional ornithologists have a weak knowledge of scientific names, and
even fewer are inclined to delve into the general history of ornithology in
North America on their own. An English
name, however, gets the attention of every bird person who uses it. Most will never take the time to read about
these people, but those who do will hopefully be inspired and will be reminded
that we got where we are today in our understanding of birds through the work
of those who preceded us. Knowing that we
humans are instinctively interested in stories about other humans, I doubt that
I am the only one inspired by the stories of people like Brewster. Some of you may know why I have a special admiration
for Brewster, but I hope all of you will appreciate this accomplishments and
the reverence in which he was held by his peers. Contrast the window into ornithology opened
by “Brewster’s Booby” compared to the value of a name like “Brown Booby,” its
sister species. Yes, Brown Booby is an
excellent descriptive name. It is indeed
a brown booby, brownest of the genus.
But that’s where the value ends.
There is no depth, no cachet, no inspiration. In my opinion, I think that having a tiny
percentage of English names that honor contributors to ornithology acknowledges
that we did not get here on our own and provides potential inspiration for
others to contribute to ornithology and especially in a case like Brewster to
appreciate birds more deeply.
“Additional
points: one problem with Cocos is that the name is clearly associated with
three endemics of Cocos Island (Cocos Cuckoo, Cocos Flycatcher, Cocos Finch)
and thus the natural implication of the name “Cocos Booby” is that it is
another island endemic. Few people are
aware of the name Cocos Plate, and so confusion will be inevitable. By the way, there is no need to discuss
adding group names in this case, e.g. “Cocos Brown-Booby” etc., because our
guidelines indicate that there is no need for that if two species previously
split, then lumped, and then re-split --- the original species treatment used
Brewster’s Booby for S. brewsteri (and White-bellied Booby for S.
leucogaster). Using White-bellied
Booby for narrowly defined S. leucogaster also should be discussed, as
recommended in our guidelines on English names because it calls attention to
the species split and restores a historical name used in much literature prior
to 1957.”
Comments from Don Roberson (voting for Claramunt on
Part B): YES on Cocos Booby.
Although "Brewster's Booby" would have been okay with me, some people
disfavor eponymous common names, so a different English name is fine at this
point in time. The genus Sula
currently has a nice set of English names: Red-footed, Blue-footed, Brown,
Peruvian, Masked, and Nazca. [in the Sulidae, only the monotypic genus Papasula
has an eponymous name, but it resides far from the New World.]
“Traditionally, the long-standing set of
the five basic Sula boobies were Red-footed, Blue-footed, Brown,
Peruvian, and Masked. All are short, distinctive, and memorable -- 4 are based
on plumage and/or soft-parts color, and one is named for the Peruvian Current
where it occurs. Red-footed and Blue-footed are surely among the "really
good English names" for those species. Brown and Masked are accurate and
serviceable. Then came the first split in Sula, and I was delighted when
the authors of the taxonomic paper (Pitman & Jehl 1998) concluded with this
line: "As a common name for Sula granti we propose Nazca
Booby, which recognizes that the current breeding range and probably
evolutionary history of this species is closely associated with the Nazca
Crustal Plate."
“This was a great English name -- short,
very memorable, reasonable accurate, and innovative. In 1989, when I spent 4
months in the eastern tropical Pacific on a NOAA ship, with Bob Pitman on a
sister ship on that cruise, Bob asked me to take notes on the juvenal
"Masked" Boobies when we went through the Galapagos, which he used in
writing his 1998 paper [later, I wrote a paper on ID issues in Masked &
Nazca Boobies.] On that same cruise, we had the good fortune to visit Isla del
Coco [Cocos Island, off Costa Rica] and even land on the island. My notes also
mention a "breeding colony on Nuez I., where
Gary Fredrichsen saw fledglings" of brewsteri
Brown Booby while I was on Cocos itself (Nuez is a
tiny islet in Chatham Bay; we also scoped it with the ship's Big Eyes).
“Now, we have an opportunity to give S.
brewsteri another excellent English name: Cocos Booby. It parallels the
name Nazca Booby nicely. Neither name includes all the breeding range of the Sula
involved, let alone its at-sea distribution, but
Cocos is a core breeding location, well represents core at-sea range, and does
so better than the Nazca Plate did for Nazca Booby. It is really a fine choice:
short, very memorable, reasonable accurate, and innovative in parallel with
Nazca. While it is useful to name local resident landbird endemics for the
island on which they occur (e.g., Cocos Finch), it is also appropriate to name
a seabird after the tectonic plate which covers a good portion of both its
breeding and its pelagic range, postulating, as did Pitman & Jehl, that it
is likely that the "evolutionary history of this species is closely
associated with the Cocos Crustal Plate."
“None of the other options are as
attractive. "White-headed" and "Pale-headed" are rather
boring, do not accurately describe all subspecies, and focus only on adult male
plumages but would not be accurate for well over half of the birds encountered
(females, sub-adults, and juvs). "Tropical Eastern Pacific Booby" is
a jaw-breaker, not significantly more accurate, and would break the string of
having the names of all Sula boobies be short, distinctive, and punchy.
Finally, "Mexican Booby" is misleading is multiple ways -- Mexico
(including the western coast of Mexico) has multiple species of boobies, but
more importantly, the eastern coast of Mexico will have a Brown Booby from the
Gulf that is not brewsteri, so a "Mexican Booby" would not
even include the Caribbean coast, with its entirely different type of
"Brown Booby", adding to confusion.
“VanderWerf et al. (2023) did point out
that there are four major lineages in Brown Booby: Caribbean, central Atlantic,
Indo-central Pacific, and eastern Pacific. Some have suggested that all might
be elevated to species level, and that in doing so, a hyphenated English name
-- Brown-Booby -- should be preceded by a modifier to create, say, Caribbean
Brown-Booby, Atlantic Brown-Booby etc. SACC has adopted this sort of scheme
multiple times, but generally in complex situations (e.g., the Trogonidae,
Thamnophilidae). The Sula boobies are currently just 7 species (with brewsteri)
and only 10 species if the other 3 Brown Booby lineages were to be split, and
even the entire Sulidae would be just 14 species. I don't think we need
hyphenated English names is this relatively small group of Sulids, and to do so
would create long names which are neither short, memorable, or punchy. But even
if later taxonomic changes might be made, Cocos Booby or "Cocos
Brown-Booby" would still be the best choice, in my opinion, for the
eastern Pacific taxa.
“Citation: Pitman & Jehl (1998)
Geographic variation and reassessment of species limits in the "Masked
" boobies of the eastern Pacific Ocean. Wilson Bull 110: 155-170.”
Comments from Claramunt (Part A): “A. YES. The combination of morphological, genetic and behavioral
information provides compelling evidence for the species status of E Pacific
taxa.”
Comments from Stiles: “A. YES to
Split Sula brewsteri from S. leucogaster. B. NO. I definitely
prefer Brewster’s as the E-name, given my strong objection to AOS’s decision to
abolish all eponyms, thus ignoring nomenclatural history and Brewster’s
significant contributions to the development of American ornithology.”
Comments from Robbins:
“A. YES for recognizing Sula brewsteri as a species given that this
taxon appears to be consistent with species-level treatment of other taxa in Sula.”
Comments from Areta: “A.
YES,
the evidence is more fitting with species status for brewsteri, although I would not be averse to having more genetic
information on the distinction and effect of gene flow between the syntopic
birds.”
Comments
from David Ainley (voting for Del-Rio): B. NO on Cocos
Booby and YES on something else. Cocos too geographically restrictive for the
species. Brewster's Brown Booby would be fine with me, and kind of how I have
always considered it. For what it's worth, though, if given the choice, I'd go
with Eastern Pacific BB (for S. brewsteri) should the other BBs become
known as Indo-Pacific BB and Atlantic BB. That's more in line with how Gannets
are named.”
Comments from Bonaccorso: “A. YES. All the evidence
supports the idea that Sula
brewsteri is a good
species, morphologically diagnosable and reproductively isolated.”
Comments from Jaramillo:
“A – YES to the split of
Brown Booby. Apart from the biology of sympatry and avoidance of hybridization
with is key here. I will also point out, and this is in the proposal that for
years it has been stated that the females are unidentifiable. But VanderWerf et
al. have shown that they are actually identifiable -- it is just more difficult. We have started
using these criteria in the field, and they seems to work.
“B – YES to Cocos Booby. Memorable, easy to spell, and a name that
is similar in etymology to Nazca. Cocos means a lot of things, including
coconuts in most Spanish places, but it is slang for testicles in Chile. Most
words are slang for testicles in Chile, so I would not be concerned, and this
is the English Name, not the Spanish Name. But I wanted to mention that. It
also can mean your brain/mind in slang.”
Comments
from Debra Shearwater (voting for Bonaccorso): “YES to
Cocos would be my vote. Memorable, easy to spell, short. It bookends with Nazca
Booby.”
Comments
from Lane:
“YES on both. It seems NACC has already accepted this split and the English
name “Cocos Booby.” The assortative nesting certainly suggests that the S.
brewsteri is acting as a good species where it overlaps with other
populations of S. leucogaster in the central and eastern Pacific. The
English name “Cocos,” whereas calling to mind a single island to me, follows
the logic of “Nazca Booby” and thus makes sense after some thought.”
Comments
from Scott Terrill: B:”NO to Cocos Booby. In voting no
on B, I agree with all of the rationale provided, in detail, by Remsen,
including but not limited to: (1) The extensive historical precedence for
the name "Brewster's" Booby with eastern Pacific taxa; (2) Brewster's
immense early contributions to ornithology and, as Remsen put it; (3)
"Henshaw made it clear that Brewster was what we would call a 'good guy'
nowadays."
Additional
comments from Stiles:
“Here, I have a very strong opinion that “Cocos Booby” is a singularly
inappropriate name for Sula brewsteri, and that the reasons for NACC’s
adoption of it are most ill-founded. To begin with, geography. The majority and
best-known part of the Cocos plate constitutes the floor of the Caribbean,
which immediately causes confusion by implying that brewsteri is a
Caribbean bird. By contrast, Cocos I. is near the western extreme of this
plate, effectively an outlier as well as constituting only a minute fraction of
the distribution of brewsteri. Regarding the presence of this species on
Cocos I., my experience suggests that it is also an insignificant part of this
distribution. I spent a week in 1975 on and around Cocos and, mindful of a
previous unconfirmed record of its breeding there on an outlying islet at about
the same time of year, I made a special effort during two slow circuits of the
island to observe this species on these islets as well as the many cliffs of
the island itself – and never saw it there, or in the surrounding waters. My
conclusion was that if brewsteri breeds (or even occurs) there, it must
be in small numbers and probably not regularly. All of this puts Cocos Booby in
the same league for aptness in distributional terms as Tennessee Warbler or
Philadelphia Vireo. The analogy between Cocos Booby and Nazca Booby (for S.
granti), while sounding cute, is also flawed (simply compare the data for
the distribution and status of brewsteri above with that of S. granti
with respect to the Nazca plate, the distribution of which lies entirely
offshore in the Pacific, the rest being subducted under the South American
continent. The major and best-studied breeding colony of granti is
centered on this plate and its seaward distribution is also centered on and
around it, making Nazca Booby a most appropriate name. In fact, the seaward
distribution of brewsteri also includes the Nazca plate (to a greater
extent than its occurrence on the Cocos plate: it could even be called the
“Lesser Nazca Booby” more appropriately). Finally, regarding English names,
Brewster’s Booby has a considerable track record among the seabird fraternity,
which after all has a better appreciation of its global distribution (than at
least, the NACC, which due to its recently-acquired obligatory eschewing of
eponyms, must ignore “Brewster´s Booby”. I see no reason why SACC should follow
suit and condone, much less honor this practice and strongly suggest that
members of the SACC approving “Cocos Booby” should reconsider their votes and
give more serious consideration to “Brewster´s Booby” as a better name.
Following the leader out of “loyalty” to the NACC seems unwise if the “leader”
itself, makes what from any knowledgeable viewpoint is an unwise decision.”
Additional
comments from Remsen:
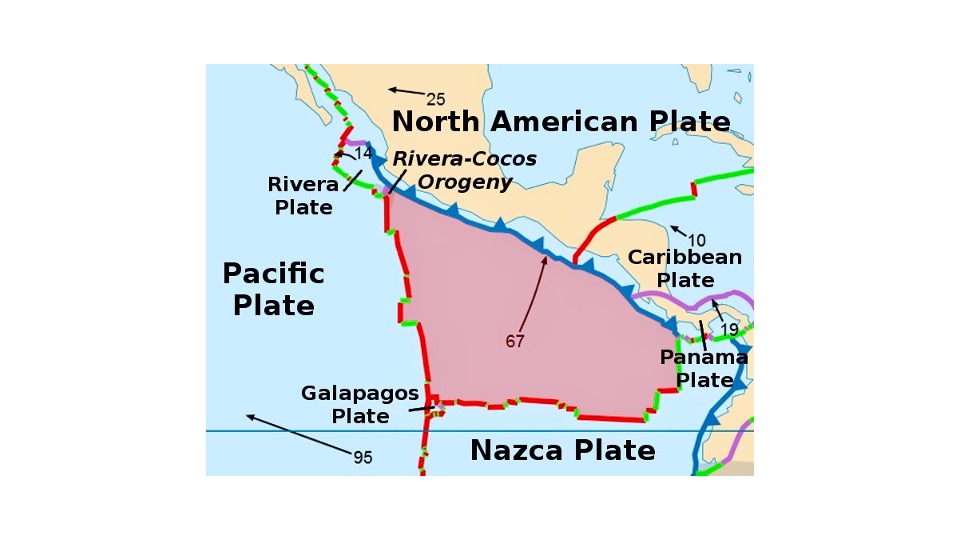
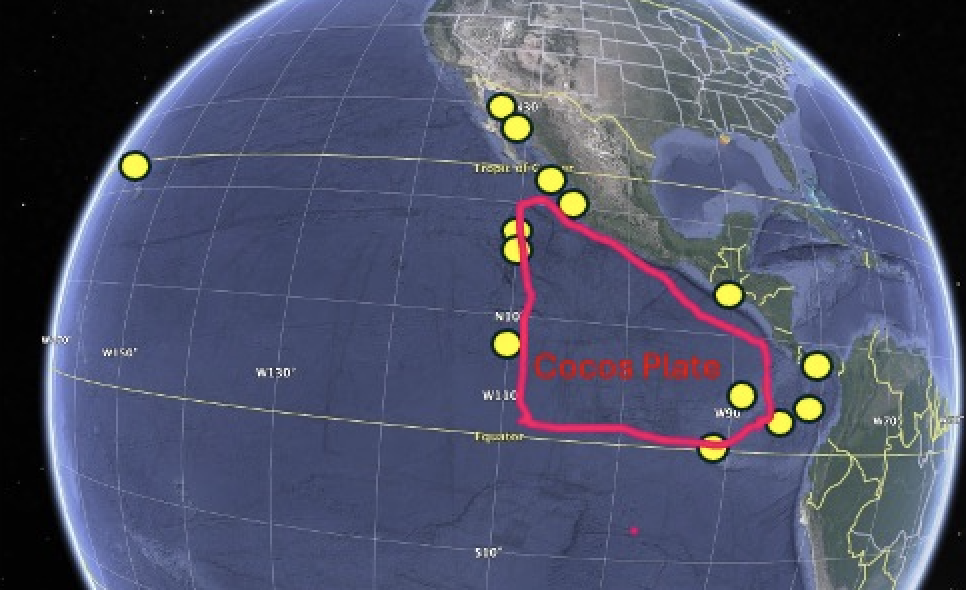
The proposal maps the Cocos Plate, and I decided to elaborate by mapping
brewsteri breeding distribution on the plate in a crude way.
Here’s
a more detailed map of the Cocos Plate from Wikipedia:
And
here’s my first attempt at crudely mapping by eye the breeding distribution of brewsteri
from some of its most prominent breeding locations as given in Birds of World
and elsewhere (yellow circles). I will
likely refine this to make it more accurate, and additions/corrections are
welcome. But the bottom line, barring
gross inaccuracy, is that for geographic name, Cocos Booby is pretty good in
that it looks like the tectonic activity of the plate produced many of the
islands on which the species breeds, and even some of the islands not on the
plate were formed by interactions between the plate and other ridges. Much of the distribution in Mexico lies north
of the plate and of course the range expansion to Hawaii has added territory
far from the plate, but nonetheless for a geographic name that doesn’t refer to
a single island or isolated mountain range, it’s not bad at all in my
opinion. The species occurs mainly along
the margins of the plate, not the deep ocean within the plate.
Comments
from Zimmer:
“YES to Part A. The clincher for me is
that both brewsteri and plotus are expanding their ranges,
increasingly coming into contact, and are still, for the most part,
assortatively mating in contact zones.
The suggested mechanism of mate choice based upon gular pouch coloration
as an indicator of fitness, and reported increased female aggression towards
“foreign” males closer to contact zones, is pretty inspired in my opinion. The situation also draws some obvious
parallels with the Blue-footed versus Peruvian boobies and the Masked versus
Nazca boobies, as noted in the Proposal.
NO on Part B. I too, prefer
reverting to the widely recognized and historically used “Brewster’s Booby” for
all of the reasons spelled out by Van and Gary.
I don’t have any particular objection to “Cocos Booby” for brewsteri,
even if it means we would be combining sexualized slang for testicles and
breasts from two different language systems in the same bird name (!) – as
others have noted, it is pithy and memorable, and has at least some precedence
in the name already given to S. granti.
However, the rationale for discarding a long-standing name with
historical traction, that honors one of the giants of ornithology, and someone
who seemingly meets the character test of even modern generations, simply to
follow what I consider to be the misguided dictates of the AOS, just doesn’t
sit well with me. Don Roberson gives a
good synopsis of why some of the other proposed names (“Mexican Booby”,
“White-headed Booby”, “Pale-headed Booby”) are not good choices, and I agree
with his reasoning on each of those. So,
I won’t fight “Cocos Booby” if that’s the way the SACC vote goes down, but I
would prefer “Brewster’s Booby” for brewsteri.”